Influence of Seasonal Variation and Growth Conditions on Seed Germination of Ebolo (Crassocephalum crepidioides and Crassocephalum rubens) Vegetables
| Received 10 Jun, 2025 |
Accepted 15 Aug, 2025 |
Published 31 Dec, 2025 |
Background and Objective: Crassocephalum crepidioides and C. rubens are plants indigenous to tropical Africa. They are important orphan crops that are highly nutritious but are still mainly harvested from the wild. One major challenge that limits the cultivation of these crops is the high degree of seed dormancy, which makes it difficult to propagate through seeds. This study aimed to investigate the effects of growth conditions, media, and seasonal variation on the seed germination capacity of C. crepidioides and C. rubens. Materials and Methods: Crassocephalum crepidioides and C. rubensseeds were cultivated in the greenhouse across two growing seasons: Winter and summer. Seed germination tests were conducted using two different growth media: Filter paper and half-strength Murashige and Skoog (½MS) medium. Seeds were incubated at varying temperatures (21 and 25°C), light intensities (30, 80 μmoL/m2/s-1), in the dark, and long-day photoperiod (16 hrs of light/8 hrs of darkness). Results: Both species exhibited a high degree of seed dormancy in the dark, but light and temperature promoted germination, with light having a stronger effect on seed germination of C. rubens. Germination was faster on filter paper for both species compared to ½MS medium. Notably, C. rubens seeds harvested in the winter exhibited higher dormancy than C. crepidioides, while summer-harvested C. crepidioides seeds showed increased dormancy compared to C. rubens. Conclusion: Overall, C. crepidioides exhibited higher dormancy compared to C. rubens and required light and temperature to break out of dormancy. Improved seed germination was observed on filter paper, while seasonal variation influenced the dormancy level in Crassocephalum seeds.
| Copyright © 2025 Adebimpe Nafisat Adedeji-Badmus. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Crassocephalum crepidioides and C. rubens are underutilized, traditional leafy vegetables indigenous to tropical Africa and widely distributed across the tropical and sub-tropical regions of the world. They belong to the plant family Asteraceae and are annuals that grow rapidly and thrive well in marginal conditions1. Both species are highly nutritious and are used as leaf vegetables and medicinal plants in Sub-Saharan Africa and Asia2,3. Despite their importance as a nutritious food source, Crassocephalum species are not commonly cultivated, but are primarily collected from the wild. One of the key challenges limiting their cultivation is a high degree of seed dormancy, which makes propagation through seeds difficult. Crassocephalum seeds have been established to require light for germination4,5. Adedeji-Badmus et al.5 reported that C. crepidioides seeds have a higher dormancy depth than C. rubens seeds, which was linked to a higher abscisic acid (ABA) level in C. crepidioides seeds at harvest. The role of ABA and Gibberellins (GA) in seed dormancy has been established. While ABA regulates seed dormancy and maintenance positively, GA promotes seed germination6,7. De novo synthesis of ABA during seed development and maturation plays a significant role in seed dormancy and germination8. Seed dormancy appears at the end of the seed maturation, where the cell cycle ceases, molecular dependence from the mother plant disappears, dehydration occurs, storage products are synthesized, and ABA is accumulated9. The ABA is the major internal physiological factor that induces seed dormancy10. It induces primary dormancy, which is an adaptive trait in seeds to prevent vivipary, and after seed dispersal, delays and spreads germination over time11,12. The level of ABA at maturity influences the seed dormancy level.
Seed dormancy and germination capacity can be significantly influenced by growth conditions and environmental variations during plant growth and development, and at harvest13. Environmental cues such as temperature, photoperiod, and drought stress can influence the germination characteristics of seeds during their development, maturation, and after dispersal. These factors may also affect the conditions under which dormancy is released14,15. Numerous studies have investigated how day length, light quality, temperature, water availability, and nutrients influence the level of dormancy in many species16-18. The duration of day length during the final stages of seed maturation can affect seed behaviour19. Some species may exhibit higher germination capacity when grown under short day conditions20,21. While day length had no significant impact on seed performance of some species22,23. Additionally, light quality during seed maturation has a direct effect on seed dormancy13. The maternal light environment during seed development plays a crucial role in determining seed weight, germinability, and longevity22. Temperature during seed development and maturation also influences seed germination or dormancy in many species16,23-28. Seeds developed at warmer temperatures are generally less dormant at maturity compared to those developed at cooler temperatures13,16,23,27,29,30.
Crassocephalum plants grow in various regions across the world and have the potential to serve as an important food crop. A major limitation of their commercial cultivation is the high degree of dormancy and low germination capacity of their seeds. The ecological conditions in which the mother plants grow can influence the dormancy level and germination capacity of Crassocephalum seeds. Thus, the present investigation examined the effect of environmental conditions and seasonal variation on seed dormancy and germination capacity of Crassocephalum seeds.
MATERIALS AND METHODS
Study area and duration: The greenhouse experiment was conducted at the Technical University of Munich (TUM) School of Life Sciences Plant Facilities in Freising, Germany (48°24'N, 11°45'E). The experiment took place over two years, during the winter of 2018/19 and the summer of 2020.
Plant material and growth conditions in the soil: Crassocephalum crepidioides accession Ile-Ife (C.C.Ile-Ife) and C. rubens accession Mali (c.r.Mali) were previously described by Rozhon et al.31 and Schramm et al.32. For soil cultivation, soil substrate C700 with Cocopor® (Stender AG, Schermbeck, Germany) was used. Crassocephalum crepidioides and C. rubens plants were grown in the greenhouse at two different seasons (winter and summer). Seeds were harvested during the 2018/19 winter and 2020 summer periods. Plants were initially cultivated in growth chambers (Bright Boy, CLF Plant Climatics, Wertingen), equipped with Philips Master TLD 58W/840 light bulbs. The standard growth conditions were a temperature of 25±2°C and cycles of 16 hrs of white light and 8 hrs of darkness, with an intensity of 80 μmol m-2s-1. Plants were germinated and pre-grown in small pots, filled with soil substrate, in the growth chambers for 3-4 weeks, using the conditions above, and then transferred to larger pots in the greenhouse. In the greenhouse, the plants were grown at a temperature of 20±2°C, 50% relative humidity with artificial lightning (80-100 μmol m-2s-1 for 12 hrs) in winter and 25±3°C, 50% relative humidity without artificial lightning during the summer in Freising, Germany (48°24'N, 11°45'E). Watering was done with tap water; no additional fertilizer was added.
Germination experiments: Crassocephalum seeds were sterilized using 75% commercial bleach solution containing 0.01% Triton X-100. The bleach solution was added to the seeds and then gently shaken on the bench for 20 min. Seeds were then rinsed three times with deionized water in a sterile hood and transferred to either wet filter paper (Roth, Karlsruhe, Germany) or half-strength Murashige and Skoog (½MS) medium (Duchefa, Haarlem, Netherlands). Seeds from the mother plants grown at the same time and in the same conditions were used. The seeds were incubated at different temperature regimes (21 and 25°C), light intensities (30, 80 μmol m-2s-1), in the dark, and long-day photoperiod (16 hrs of light/ 8 hrs of darkness). Germination was assessed by the emergence of the radicle from the seed coat.
RESULTS
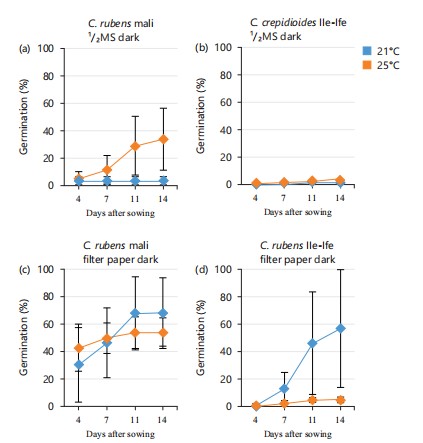
Response of C. crepidioides and C. rubens seeds to dormancy under varying growth conditions: Germination experiments were conducted on seeds of C. rubens ecotype Mali and C. crepidioides ecotype Ile-Ife. The seeds used have the same growth history. On ½MS medium, both species exhibited a high level of seed dormancy in the dark (Fig. 1a-b). While neither C. crepidioides nor C. rubens seeds showed any germination capacity in the dark at 21°C, C. rubens seeds germinated with approximately 30% efficiency at 25°C (Fig. 1a). Notably, when seeds were germinated on filter paper in the dark and at 21°C, both C. rubens and C. crepidioides seeds achieved germination efficiencies of 65 and 60%, respectively (Fig. 1c-b). Additionally, 50% germination efficiency was recorded on C. rubens when the temperature was increased to 25°C, however, raising the temperature to 25°C did not improve seed germination of C. crepidioides on filter paper (Fig. 1b). These results suggested that apart from the light requirement for the germination of Crassocephalum seeds, temperature and growth media also affect seed germination of Crassocephalum.
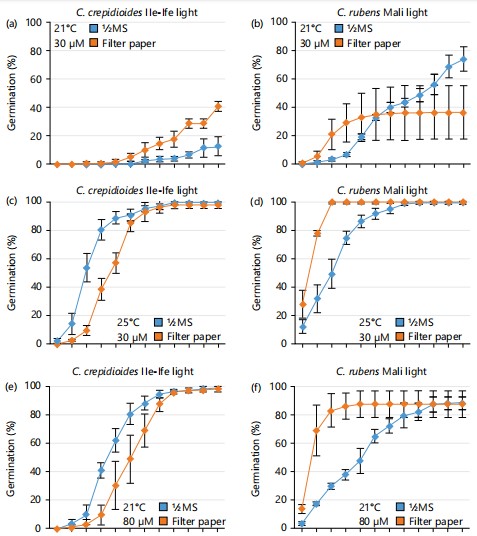
The effect of growth media on seed germination and dormancy of both C. crepidioides and C. rubens under light conditions revealed the interplay between light, temperature, and media and how these factors significantly influenced the germination of both Crassocephalum species. At 21oC and 30 μmol m-2s-1 of light, about 40% germination was observed for C. crepidioides sown on filter paper as compared to 10% germination that was recorded on ½MS at 14 days after sowing (DAS; Fig. 2a). Interestingly, C. rubens achieved about 70% germination on ½MS compared to filter paper where about 35% germination was recorded (Fig. 2b). Keeping the light intensity constant and increasing the temperature to 25°C, seed germination significantly improved on both ½MS and filter paper for both C. crepidioides and C. rubens. C. crepididoides seeds had a faster germination rate on ½MS, recording 80% germination in just 6 days as compared to filter paper, where 40% germination was recorded at 6 DAS (Fig. 2c). On the contrary, C. rubens seeds had a faster germination rate on filter paper recording 100% germination in 5 DAS whereas, a significant reduction was observed on ½MS where 50% germination was recorded within the same period (Fig. 2d).
|
In addition, the effect of increased light intensity from 30 to 80 μmol m-2s-1 on the germination outcome of both Crassocephalum species was assessed. Keeping the temperature at 21°C, there was a significant improvement in seed germination for both C. crepidioides and C. rubens compared to results obtained under 30 μmol m-2s-1. C. crepidioides seeds recorded 60% germination on ½MS at 7 DAS as compared to filter paper where approximately 30% germination was recorded under the same condition (Fig. 2e). For C. rubens, 90% germination was recorded on filter paper at 7 DAS, in contrast to ½MS where 50% germination was recorded 7 DAS (Fig. 2f). When temperature was increased to 25°C, C. crepidioides exhibited a similar germination response on both ½MS and filter paper, recording approximately 50% germination in 7 days (Fig. 2g). In contrast, C. rubens had a faster germination rate on filter paper, reaching 90% germination in 5 DAS, while only approximately 20% germination was recorded on ½MS during the same period (Fig. 2h).
Investigating the influence of environmental variations during seed development on the germination of Crassocephalum seeds, two different seed batches from C. crepidioides and C. rubens mother plants grown either during the 2018/19 winter or the 2020 summer period were subjected to germination tests. A high level of variation was observed between the germination outcomes of the two different seed batches.
Germinating the seeds at a light intensity of 30 μmol/m2/sec and at 21°C, both C. rubens and C. crepidioides seeds from mother plants grown in winter were more dormant than those from mother plants grown in summer (Fig. 3a-b). This was evident with the reduced germination capacity recorded from the seeds harvested in winter. Notably, the germination capacity of C. crepidioides seeds obtained during winter was considerably lower, with approximately 10% germination recorded 14 DAS (Fig. 3b).
 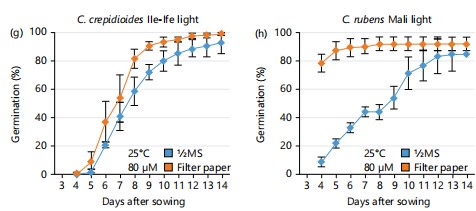
|
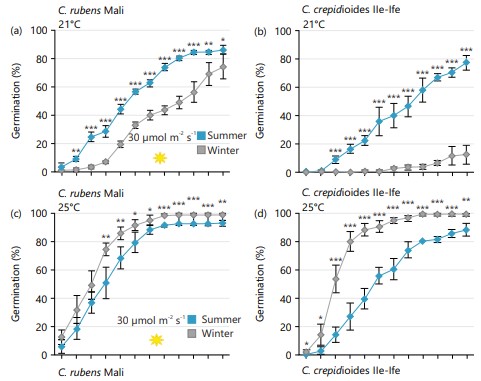 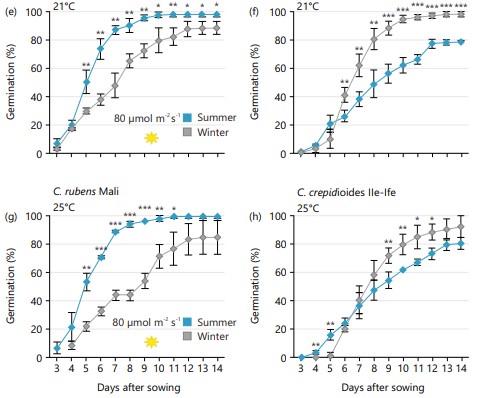
|
At higher light intensity of 80 μmol m-2s-1 or higher temperature of 25°C, the seeds were relieved of the observed dormancy (Fig. 3c-h). Interestingly, C. crepidioides seeds from plants grown in winter showed a higher germination capacity at higher temperatures and light levels than those from summer grown plants (Fig. 3d-f and h). In contrast, C. rubens seeds from mother plants grown in summer generally exhibited better germination than those from winter-grown plants. These findings are consistent regardless of the germination environment.
DISCUSSION
Ensuring food and nutritional security for a global population projected to reach approximately 10 billion by 2050 is a significant challenge. This challenge is compounded by the need to maintain crop production amid changing climatic conditions, increasing severity of abiotic and biotic stresses, and the limitations imposed by restricted agricultural land. In 2023, about 733 mL people were reported to face chronic hunger, a staggering increase from 613 mL in 201933. Additionally, an estimated 2.33 billion people were classified as moderately or severely food insecure, with Africa presenting around 282 mL undernourished people33,34. Projections indicate that, without significant intervention, achieving zero hunger by 2030 will be extremely difficult. Many underutilized crops, which are currently grown on a small scale in hunger-prone areas, have the potential to address food and nutrient deficiencies35. When integrated with staple crops, these underutilized crops could play a crucial role in meeting the nutritional needs of a growing population.
Crassocephalum crepidioides and C. rubens are two underutilized leafy vegetables with the potential as food crops36-39. They are highly nutritious and have potential as vegetables and medicinal plants. However, these crops are limited in cultivation and are primarily harvested from the wild. The major constraint limiting the cultivation is high degree of seed dormancy. Light efficiently promoted germination of C. crepidioides and C. rubens seeds5. The effect of light on seed germination of C. crepidioides and C. rubens had already been studied, and it was reported that both species required light for germination4,5. Adedeji-Badmus et al.5 established that C. crepidioides seeds are highly dormant as compared to C. rubens. This was due to the high amount of ABA in C. crepidioides seeds at harvest. The requirement of light for C. crepidioides surpasses that of C. rubens. This was evident from the germination experiment in the dark. No germination was recorded for C. crepidioides on ½MS media, irrespective of the temperature used, compared to C. rubens. A similar observation was reported by Adedeji-Badmus et al.5. Similarly, C. crepidioides recorded low germination on filter paper compared to C. rubens.
Light and temperature promote germination of C. crepidioides and C. rubens. Low temperature (21°C) and low light I ntensity (30 μmol m-2s-1) significantly impact seed germination of both C. crepidioides and C. rubens. High temperature (25°C) and low light intensity (30 μmol m-2s-1), low temperature (21°C) and high light intensity (80 μmol m-2s-1); and high temperature (25°C) and high light intensity (80 μmol m-2s-1) significantly improved seed germination of both C. crepidioides and C. rubens. This indicates that there is an interplay of light and temperature in the seed germination of C. crepidioides and C. rubens. Adedeji-Badmus et al.5 also observed a significant improvement in seed germination of C. crepidioides and C. rubens when the temperature was increased to 25°C. Yuan and Wen4 recorded the highest germination for C. crepidioides at 25°C and 25 μmol m-2s-1.
Considering the effect of media on seed germination of C. crepidioides and C. rubens, it was observed that filter paper promoted seed germination of both species in the dark. However, under light conditions, both ½MS and filter paper promoted seed germination. Under low light (30 μmol m-2s-1) and low temperature (21°C) conditions, filter paper promoted seed germination of C. crepidioides while ½MS media was more effective for C. rubens. This indicates that, in addition to light and temperature, the choice of growth media significantly influences the seed germination of these Crassocephalum species. At a higher temperature (25°C) and light intensity (80 μmol m-2s-1), ½MS improved the speed of germination in C. crepididoides. Conversely, for C. rubens, the speed of germination improved on filter paper.
Growth conditions at harvest and seasonal variation during the growth and development of plants can have a significant impact on the dormancy level and germination capacity of seeds. Therefore, it is important to assess the effect of seasonal variation and growth conditions on the seed germination capacity of Crassocephalum seeds. According to Baskin and Baskin18, the environmental conditions under which seeds develop can influence seed quality. In this study, both C. rubens and C. crepidioides seeds harvested from the mother plants grown during winter were more dormant than those harvested from mother plants grown in summer. The effect of seasonal variations on seed dormancy and germination was particularly noticeable when Crassocephalum seeds were germinated under high temperatures or high light intensities. Specifically, C. crepidioides seeds from summer grown plants were more dormant than the winter-grown plants. Conversely, C. rubens seeds from winter-grown plants displayed a higher dormancy level than those from the summer-grown plants.
Environmental conditions during seed maturation, such as photoperiod and temperature, have a strong influence on seed dormancy and germination14,18,40. Temperature variation during seed set and maturation strongly affects seed dormancy13. Generally, lower temperatures almost always result in lower germination. Interestingly, C. crepidioides seeds from summer-grown plants have lower germination capacity as compared to the winter-grown plants. However, seed produced by summer-grown C. rubens plants tends to have a higher germination capacity.
CONCLUSION
In conclusion, there is an interplay between light, temperature, and growth media on seed germination of C. crepidioides and C. rubens. Generally, C. crepidioides exhibited higher dormancy compared to C. rubens and required light and temperature to break out of dormancy. Low temperature and low light impaired seed germination of both species. The choice of germinating media also influences the speed and rate of germination of Crassocephalum seeds. The ½MS significantly improved seed germination of C. crepidioides, while filter paper was more effective for C. rubens. Additionally, growth conditions and seasonal variation during maternal plant growth influenced the dormancy level in Crassocephalum seeds. Typically, C. rubens seeds harvested in winter exhibited higher dormancy than those harvested in summer, while C. crepidioides seeds harvested in the summer showed higher dormancy compared to the winter-harvested seeds.
SIGNIFICANCE STATEMENT
This study identified the optimal environmental conditions light, temperature, and media type required to overcome seed dormancy in Crassocephalum crepidioides and C. rubens, which could be beneficial for enhancing their cultivation and supporting efforts toward their domestication. The study will assist researchers in uncovering critical areas of seed physiology and dormancy regulation that have remained unexplored by many. Consequently, a new theory on photothermal and substrate dependent dormancy mechanisms in tropical leafy vegetables may be developed.
ACKNOWLEDGMENTS
Special appreciation goes to Prof. Dr. Brigitte Poppenberger for her invaluable insights andhelpful discussions throughout this project. The author also appreciates the Millennium Seed Bank at Kew Royal Botanic Gardens for the seeds of the Crassocephalum accessions used. Special thanks go to Irene Ziegler and the horticultural staff of the TUM School of Life Sciences Plant Facilities for their technical support.
REFERENCES
- Dairo, F.A.S. and I.G. Adanlawo, 2007. Nutritional quality of Crassocephalum crepidioides and Senecio biafrae. Pak. J. Nutr., 6: 35-39.
- Dansi, A., A. Adjatin, H. Adoukonou-Sagbadja, V. Falade, H. Yedomonhan, D. Odou and B. Dossou, 2008. Traditional leafy vegetables and their use in the Benin Republic. Genet. Resour. Crop E, 55: 1239-1256.
- Dansi, A., R. Vodouhè, P. Azokpota, H. Yedomonhan and P. Assogba et al., 2012. Diversity of the neglected and underutilized crop species of importance in Benin. Sci. World J., 2012.
- Yuan, X. and B. Wen, 2018. Seed germination response to high temperature and water stress in three invasive Asteraceae weeds from Xishuangbanna, SW China. PLoS ONE, 13.
- Adedeji-Badmus, A.N., S. Schramm, M. Gigl, W. Iwebema and P. Albertos et al., 2022. Species-specific variation in abscisic acid homeostasis and responses impacts important traits in Crassocephalum orphan crops. Front. Plant Sci., 13.
- Kucera, B., M.A. Cohn and G. Leubner-Metzger, 2005. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res., 15: 281-307.
- Finkelstein, R., W. Reeves, T. Ariizumi and C. Steber, 2008. molecular aspects of seed dormancy. Annu. Rev. Plant Biol., 59: 387-415.
- Kermode, A.R., 1995. Regulatory Mechanisms in the Transition from Seed Development to Germination: Interactions Between the Embryo and the Seed Environment. In: Seed Development and Germination, Kigel, J. and G. Galili (Eds.). Marcel Dekker, Inc., New York, pp: 273-332.
- Cadman, C.S.C., P.E. Toorop, H.W.M. Hilhorst, W.E. Finch‐Savage, 2006. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J., 46: 805-822.
- Gutierrez, L., O. van Wuytswinkel, M. Castelain and C. Bellini, 2007. Combined networks regulating seed maturation. Trends Plant Sci., 12: 294-300.
- Kermode, A.R., 2005. Role of abscisic acid in seed dormancy. J. Plant Growth Regul., 24: 319-344.
- Sano, N. and A. Marion-Poll, 2021. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. mol. Sci., 22.
- Penfield, S. and D.R. MacGregor, 2017. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot., 68.
- Donohue, K., 2009. Completing the cycle: Maternal effects as the missing link in plant life histories. Phil. Trans. R. Soc. B, 364: 1059-1074.
- Walck, J.L., S.N. Hidayati, K.W. Dixon, K. Thompson and P. Poschlod, 2011. Climate change and plant regeneration from seed. Global Change Biol., 17: 2145-2161.
- Fenner, M., 1991. The effects of the parent environment on seed germinability. Seed Sci. Res., 1: 75-84.
- Hillhorst, H.W.M., 1995. A critical update on seed dormancy I. Primary dormancy. Seed Sci. Res., 5: 61-73.
- Baskin, C.C. and J.M. Baskin, 2014. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. 2nd Edn., Academic Press, Elsevier, USA., ISBN-13: 9780124166837, Pages: 1166.
- Gutterman, Y., 2000. Genotypic and Phenotypic Germination Survival Strategies of Ecotypes and Annual Plant Species in the Negev Desert of Israel. In: Seed Biology: Advances and Applications. Proceedings of the Sixth International Workshop on Seeds, Merida, Mexico, 1999, Black, M., K.J. Bradford and J. Vazquez-Ramos (Eds.), CABI Digital Library, Boehringer-Mannheim, Germany, ISBN: 978-0-85199-404-8, pp: 389-399.
- Gutterman, Y., 1978. Germinability of seeds as a function of the maternal environment. Acta Hortic., 83: 49-56.
- Munir, J., L.A. Dorn, K. Donohue and J. Schmitt, 2001. The effect of maternal photoperiod on seasonal dormancy in Arabidopsis thaliana (Brassicaceae). Am. J. Bot., 88: 1240-1249.
- Contreras, S., M.A. Bennett, J.D. Metzger and D. Tay, 2008. Maternal light environment during seed development affects lettuce seed weight, germinability and storability. HortScience, 43: 845-852.
- He, H., D. de Souza Vidigal, L.B. Snoek, S. Schnabel, H. Nijveen, H. Hilhorst and L. Bentsink et al., 2014. Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot., 65: 6603-6615.
- Biddulph, T.B., J.A. Plummer, T.L. Setter and D.J. Mares, 2007. Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.). Field Crops Res., 103: 139-153.
- Llorens, L., M. Pons, L. Gil and H. Boira, 2008. Seasonality of seed production and germination trends of Fumana ericoides (Cistaceae) in the west semiarid Mediterranean region. J. Arid. Environ., 72: 121-126.
- Javaid, A., S. Shafique and S. Shafique, 2010. Seasonal pattern of seed dormancy in Parthenium hysterophorus L. Pak. J. Bot., 42: 497-503.
- Kendall, S.L., A. Hellwege, P. Marriot, C. Whalley, I.A. Graham and S. Penfield, 2011. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell, 23: 2568-2580.
- Z. Huang, S. Footitt and W.E. Finch-Savage, 2014. The effect of temperature on reproduction in the summer and winter annual Arabidopsis thaliana ecotypes Bur and Cvi. Ann. Bot., 113: 921-929.
- Huang, X., J. Schmitt, L. Dorn, C. Griffith and S. Effgen et al., 2010. The earliest stages of adaptation in an experimental plant population: Strong selection on QTLS for seed dormancy. mol. Ecol., 19: 1335-1351.
- Penfield, S. and V. Springthorpe, 2012. Understanding chilling responses in Arabidopsis seeds and their contribution to life history. Phil. Trans. R. Soc. B, 367: 291-297.
- Rozhon, W., L. Kammermeier, S. Schramm, N. Towfique, N.A. Adedeji, S.A. Ajayi and B. Poppenberger, 2018. Quantification of the pyrrolizidine alkaloid jacobine in Crassocephalum crepidioides by cation exchange high-performance liquid chromatography. Phytochem. Anal., 29: 48-58.
- Schramm, S., W. Rozhon, A.N. Adedeji-Badmus, Y. Liang, S. Nayem, T. Winkelmann and B. Poppenberger, 2021. The orphan crop Crassocephalum crepidioides accumulates the pyrrolizidine alkaloid jacobine in response to nitrogen starvation. Front. Plant Sci., 12.
- FAO, IFAD, UNICEF, WFP and WHO, 2024. The State of Food Security and Nutrition in the World 2024: Financing to End Hunger, Food Insecurity and Malnutrition in All its Forms. FAO, IFAD, UNICEF, WFP, WHO, Rome, Italy, ISBN: 978-92-5-138882-2, Pages: 286.
- FAO, IFAD, UNICEF, WFP and WHO, 2023. The State of Food Security and Nutrition in the World 2023: Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural-Urban Continuum. FAO, IFAD, UNICEF, WFP and WHO, Rome, Italy, ISBN-978-92-5-137226-5, Pages: 316.
- Singh, R.K., N. Sreenivasulu and M. Prasad, 2022. Potential of underutilized crops to introduce the nutritional diversity and achieve zero hunger. Funct. Integr. Genomics, 22: 1459-1465.
- Hendre, P.S., S. Muthemba, R. Kariba, A. Muchugi and Y. Fu et al., 2019. African Orphan Crops Consortium (AOCC): Status of developing genomic resources for African orphan crops. Planta, 250: 989-1003.
- Jamnadass, R., R.H. Mumm, I. Hale, P. Hendre and A. Muchugi et al., 2020. Enhancing African orphan crops with genomics. Nat. Genet., 52: 356-360.
- Hung, N.H., P. Satyal, do Ngoc Dai, T.A. Tai and le Thi Huong et al., 2019. Chemical compositions of Crassocephalum crepidioides essential oils and larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Nat. Prod. Commun., 14.
- Thakur, S., R. Koundal, D. Kumar, A.K. Maurya, Y.S. Padwad, B. Lal and V.K. Agnihotri, 2019. Volatile composition and cytotoxic activity of aerial parts of Crassocephalum crepidioides growing in Western Himalaya, India. Int. J. Pharm. Sci., 81.
- Dechaine, J.M., G. Gardner and C. Weinig, 2009. Phytochromes differentially regulate seed germination responses to light quality and temperature cues during seed maturation. Plant Cell Environ., 32: 1297-1309.
How to Cite this paper?
APA-7 Style
Adedeji-Badmus,
A.N. (2025). Influence of Seasonal Variation and Growth Conditions on Seed Germination of Ebolo (Crassocephalum crepidioides and Crassocephalum rubens) Vegetables. Asian Journal of Biological Sciences, 18(4), 817-827. https://doi.org/10.3923/ajbs.2025.817.827
ACS Style
Adedeji-Badmus,
A.N. Influence of Seasonal Variation and Growth Conditions on Seed Germination of Ebolo (Crassocephalum crepidioides and Crassocephalum rubens) Vegetables. Asian J. Biol. Sci 2025, 18, 817-827. https://doi.org/10.3923/ajbs.2025.817.827
AMA Style
Adedeji-Badmus
AN. Influence of Seasonal Variation and Growth Conditions on Seed Germination of Ebolo (Crassocephalum crepidioides and Crassocephalum rubens) Vegetables. Asian Journal of Biological Sciences. 2025; 18(4): 817-827. https://doi.org/10.3923/ajbs.2025.817.827
Chicago/Turabian Style
Adedeji-Badmus, Adebimpe, Nafisat.
2025. "Influence of Seasonal Variation and Growth Conditions on Seed Germination of Ebolo (Crassocephalum crepidioides and Crassocephalum rubens) Vegetables" Asian Journal of Biological Sciences 18, no. 4: 817-827. https://doi.org/10.3923/ajbs.2025.817.827

This work is licensed under a Creative Commons Attribution 4.0 International License.



