Phytochemical Analysis and Antioxidant Potential of Calotropis procera and Calotropis gigantea
| Received 10 Apr, 2023 |
Accepted 21 Jul, 2023 |
Published 30 Sep, 2023 |
Background and Objective: Calotropis species have several phytochemical constituents and are used for different human diseases. The major objective of the present study was to evaluate the phytochemical screening, antioxidant activity, detection of phenolic compounds and proximate analysis of Calotropisspecies. Materials and Methods: The samples of both plants were taken from Haripur and processed for analysis on different parameters. Various proximate analyses included fresh weight, dry weight, ash contents and crude fiber. The screenings of phytochemicals were done by using the analytical screening technique. The phenolic content was examined by the reported method of Ainsworth and Gillespie, using the Folin-Ciocalteu reagent. The superoxide dismutase enzyme was observed in a spectrophotometer. The statistical significance was determined at 30 with 95% significance (p≤0.05). Results: The higher phytochemical constituents in Calotropis procera include phenolic compounds, flavonoids, antioxidant activity, Catalase, peroxidase and pH. In Calotropis gigantea the higher concentration was recorded for carotenoid contents, enzyme superoxide dismutase, crude fiber and ash. Conclusion: It can be concluded that Calotropis procera has more phytochemical constituents in leaves and roots as compared to Calotropis gigantea while the floral phytochemicals of Calotropis gigantea were higher as compared to flowers of Calotropis procera.
| Copyright © 2023 Rehman et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Asclepiadaceae is a large group that contains 177-180 genera and contains approximately 2200 species and the species of this family are present in subtropical and tropical areas of the world, there are 23 genera and 41 species present in Pakistan. Genus Calotropis contains four species which consist of short trees and shrubs divided into a large number of regions or countries e.g., Pakistan, India, Afghanistan, Iran and Arab. The Calotropis has two common species one is Calotropis procera and second Calotropis gigantea. These two species are closely resembling each other and also and they have similar use may be due to their taxonomic characteristics1.
The Calotropis procera is recognized by its circular flower bud and the Calotropis gigantea flower bud is cylindrical. Their leaves were used for joint pain even in old times, the first addresses are cleaned and then applied to affected areas. Many phytochemical constituents in both of these plants are very important2. Plants have been used for medicine for thousands of years and the knowledge about the use of plants and their products by people is based on hit and trial experiences. Apart from pain and inflammation, there are diseases like cancer and infections which may increase the molecules that damage the body cells and make the normal metabolic process slow and also increase the pain3.
Calotropis is a wildly growing weed found in Pakistan and many other regions of the world. There are two common species C. procera and C. gigantea these species are different in some characteristics eventually C. gigantea leaf is covered with fine soft hairs which may be important and C. procera has less hairy4. Calotropis have several phytochemical constituents like alkaloids, terpenoids, carbohydrates and glycosides have been described. Calotropis had an important role in hepatoprotective, anticancer, antifertility and anti-inflammatory activities used for different human diseases such as skin disease, leukoderma, ulceration, boils, infectious diseases, swelling, tinea pedis, detach prickle from the body, immune diseases and Hansen's disease5. Calotropis procera is traditionally used to cure some diseases like skin diseases, intestinal worms and abdominal viscera enlargement, the latex extract of Calotropis procera root bark is used to cure these diseases6.
Calotropis gigantea have huge medicinal value in their leaves, root, stem and flower which have higher potential against many diseases i.e., earache, stress, toothache, pain, diarrhea and mental illness also used to cure burns, cuts, wounds and to stop bleeding7,8. Photochemical screening has shown the occurrence several of subsidiary metabolites in the alkaloids, tannins, flavonoids, saponin and steroids which are playing an essential role in the direction of the predation by using phytophagous, microbes and viruses, parasites, bugs, etc. The mentioned bioactive chemical compounds which are present in plants are very helpful in human health and have the ability to prevent diseases. The present study was conducted to analyze the plant species phytochemically and evaluate the plant’s species for antioxidant potential. To check the total phenolic compounds of both species. To measure health-promoting enzymes in Calotropis species.
MATERIALS AND METHODS
Observational site and collection of herbal plant: This experiment was done at the Horticulture Laboratory, Department of Horticulture, The University of Haripur. The present study was conducted during 2018-2019 at Haripur, Khyber Pakhtunkhwa Pakistan. Two species of herbal plants (Calotropis procera and Calotropis gigantea) were collected from plain areas of Haripur. After a complete survey, the mature plants were selected and the collection was done in plastic bags. After collection, the plant samples were matched with preserved specimens from the Department of Botany, Hazara University, Mansehra.
Preparation of plant samples: One-gram powder-prepared samples of both (Calotropis procera and Calotropis gigantea) were mixed in 15 mL of distilled water. The sample was prepared and mixed with the help of an electric shaker (SK-0180-S China) and filtered with the help of filter paper. Plant samples were then transferred to different bottles and storage solutions in the refrigerator for further analysis.
Determination of proximate analysis of Calotropis species: Various proximate analysis was performed in both species (Calotropis procera and Calotropis gigantea) i.e., fresh weight, dry weight, ash contents and crude fiber were determined according to the reported method of Okwu et al.8. Fresh plants of the samples were collected from the field and washed well with tap water for the separation of all dirt from both plants and weighed on a scale. Both plants were dried separately in an oven at 100°F for 75 hrs, after that both plants were cooled and weighed on a scale. Two grams of both plant samples were ignited in a muffle furnace at 550-600°C for 6 hrs and the ash contents of both plants were determined. Both plant extracts were prepared by taking 2 g of plant samples and putting 4 mL of sulfuric acid (H2SO4) in it and leaving it for 30% min after that 2 mL sodium hydroxide was mixed and the weight of both species was calculated for the presence of crude fiber. Both (Calotropis procera and Calotropis gigantea) plant extracts’ pH were measured in 1:5 water suspension with a pH meter9 KL-009(III) China.
Statistical analysis: The recorded data was tabulated theme-wise and the Microsoft Excel program was used in the calculation of values and its presentation in graphic form. The statistical significance was determined at 30% with 95% significance (p≤0.05).
Screening of phytochemicals in herbal plants: The screenings of phytochemicals were done by using the analytical screening technique. Leaves, root and flowers of Calotropis procera and Calotropis gigantea extract were prepared in methanol solution with a ratio of 1:12. Selected plants extracts were prepared by taking two grams’ powder and liquefying in 20 mL of affirmed complex and for purification samples the filter paper was used, for this the Wittman filter paper was used for the evaluation of flavonoids, diterpenes, phytosterols alkaloids, proteins, saponin, tannins and phenols. Plants extract were dissolved in dilute HCL and then filter the extract and extract were treated with Wagner’s reagent solution (I2 in potassium iodide). The appearance of brown or radish precipitates shows the existence of alkaloids. Both plant extracts were diluted with 20 mL distilled water and dissolved in a cylinder for 16 min, the appearance of a 2 cm layer of foam showing the presence of saponins. Plant extracts of 1% gelatin solution containing sodium chloride (NaCl) were mixed. The occurrence of white precipitate shows the existence of tannins. For the detection of flavonoids both (Calotropis procera and Calotropis gigantea) plant extracts were treated with two to three drops of sodium hydroxide solution. The presence of yellowish colour disappeared after the collection of dilute acid which designates the presence of flavonoids10. Both plant extracts were treated with a few drops of concentrated nitric acid (HNO3) and yellowish colour appeared which shows the occurrence of proteins11. Both plant extracts were treated with Cu (CH3COO)2 solution and dissolved in water. The appearance of bright green colour shows the presence of diterpenes12. Both plant extracts were treated with chloroform (CHCL3) and purified after that add a few drops of concentrated H2SO4 then shake and leave it for some time, the presence of golden colour indicated triterpenes13. Both plant extracts were treated with three to four drops of ferric chloride (FeCl3) solution. Appearances of bluish-black colour identify the phenols.
Quantitative analysis: The quantity of total phenolic content was examined by the reported method of Ainsworth and Gillespie, using Folin-Ciocalteu reagent, both plant extracts were prepared by taking one gram of plants sample Calotropis procera and Calotropis gigantea and adding 5 mL of extraction mixture14. Total solution of 100 mL mixed with (10 mL) FC-reagent. In all samples, FC-reagent (100 μL) was mixed and dissolved sodium carbonate (700 mM) was added and put at 25°C for 30 min. The samples (100 μL) were calculated at 765 nm. The amount of TPC was calculated using a calibration curve for gallic acid. The results were expressed as gallic acid equivalent. The total antioxidant activity was done to evaluate the scavenging abilities of 2, 2-diphenyl-1-picrylhydrazyl stable radicals. Through DPPH free radical inhibition in percent (%) was measured by the following formula:
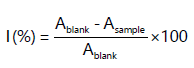 |
The flavonoid contents were measured by the reported process by Kim et al.15. Four milliliters of distilled water was mixed with 1 mL of both plant extracts measured. Then it was mixed with 5% NaNO2 solution, trail by 11% AlCl3 solution 0.4 mL. Test tubes were put on specific heat for 5-7 min and after that 2 mL of 1M NaOH was mixed and the quantity of blend was made up to 8 mL by the collection of distilled water.
The mixture was shaken and the pink colour appeared then observed at 510 nm. All the dimensions were used and mean values were observed. Both herbal plant extracts were treated with 2 mL of acetone and the solution was homogenized for 2 min and put at 4°C in darkness until it turns white, the homogenate was centrifuged at 16,000×g for 16 min and 200 μL supernatant from each tube. The absorbance was recorded at 470 nm and expressed as mg/100 g fresh weight. The absorption of total carotenoid was analyzed as follows:
Enzymes study in Calotropis procera and Calotropis gigantea: Plant extracts were prepared by taking one gram of plant samples and adding 2 mL of phosphate buffer with adjusted pH 7. After adjusting the pH, the extracts of both species were centrifuged for 3 min. The superoxide dismutase enzyme was observed in a spectrophotometer (ST-UV-755B China). The incubation medium was established and a final volume of 3.1 mL, 500 μL potassium phosphate buffer (pH 7.8), 200 μL methionine, 100 μL riboflavin, 100 μL NBT and 22 μM potassium cyanide. At 25°C the tubes were kept in an aluminum foil, prepared with 16W fluorescent lamps. Plant solution exposure to light for 15 min, the decreased NBT was measured spectrophotometrically at 560 nm. The readings were measured as unit’s mg–1 protein. Catalase activity was done through the method of Aebi and Luck. To start the reaction 1 mL mixture had 100 μL H2O2 (5.9 mM), 100 μL of enzyme extract and potassium phosphate buffer (pH 7.0). The reaction proceeded at 240 nm for 4 min and water utilization was measured using extinction of coefficient, 39.4 mM–1 cm–1. Peroxidase was done through the guaicol oxidation method. A mixture containing 800 μL potassium phosphate buffer (pH 5), 100 μL guaicol (20 mM) and 100 μL enzyme extract. With the addition of 100 μL H2O2 (40 Mm), reactions begin. An increase in absorbance was measured in 35S at 470 nm. The unit of peroxidase activity was reported with the change in absorbance per min and specific activity as enzyme units per mg soluble protein.
RESULTS
Screening of phytochemicals in Calotropis procera and Calotropis gigantea phytochemical test of both plant samples (leaves, flower and root) of C. procera and C. gigantea were collected from District Haripur. The phytochemical screening e.g., Diterpenes, tannins, protein, saponin, flavonoids, alkaloids, phenols and phytosterol were accomplished and results were recorded (Table 1 and 2).
Phytochemical screening of various parts of Calotropis procera: Various phytochemicals were examined in different plant parts i.e., leaves, flowers and roots of Calotropis procera. Alkaloids and tannins were moderately present in all parts of Calotropis procera. Saponins were highly detected in roots and leaves and moderate in flowers. Flavonoids were highly detected in flowers and leaves as compared to the root. Proteins were highly detected in leaves and roots and moderately in flowers. Diterpenes were highly reported for leaves, while absent in roots and flowers. The high phenolic amounts were present in leaves, moderately in flowers and absent in roots. Data showed that phytosterols were absent in flowers and roots and present in leaves.
Screening of phytochemicals in different parts of Calotropis gigantea: Various phytochemical compounds were examined in different parts (leaves, flowers and roots) of Calotropis gigantea. Alkaloids were moderately detected in all parts (leaves, flowers and roots). Tannins, saponins and flavonoids were highly detected in flowers. Proteins were present in flowers and leaves and absent in roots. Diterpene was highly detected in flowers and leaves and absent in roots. Phenol was moderately present in the leaves. Phytosterols were absent in the flowers of Calotropis gigantea.
| Table 1: | Phytochemicals of Calotropis procera and Calotropis gigantea from Haripur | |||
Calotropis procera |
Calotropis gigantea |
|||||
| Phytochemicals | Leaves |
Flower |
Root |
Leaves |
Flower |
Root |
| Alkaloids | ++ |
++ |
++ |
++ |
++ |
++ |
| Tannins | ++ |
+ |
+ |
+ |
+++ |
+ |
| Saponins | +++ |
+ |
+++ |
++ |
+++ |
- |
| Flavonoids | +++ |
+++ |
+ |
++ |
+++ |
- |
| Protein | +++ |
++ |
+ |
+ |
+ |
- |
| Diterpenes | +++ |
- |
- |
+++ |
+++ |
- |
| Phenols | ++ |
++ |
- |
++ |
+ |
+ |
| Phytosterols | + |
- |
- |
+ |
- |
+ |
| +: Specifies existence, ++: Moderate, +++: Highly detected and -: Absent | ||||||
| Table 2: | Comparative phytochemical study in different parts of Calotropis procera and Calotropis gigantea | |||
Calotropis procera |
Calotropis gigantea |
|||||
| Phytochemicals | Leaves |
Flower |
Root |
Leaves |
Flower |
Root |
| Phenolic compounds (mg) | 132.5 |
167.1 |
68.95 |
51.05 |
149.5 |
67.32 |
| Antioxidant activity (mg) | 61.8 |
28.3 |
42.5 |
45.2 |
32.5 |
43.9 |
| Flavonoids (mg) | 2.803 |
1.006 |
2.135 |
2.194 |
2.2 |
2.102 |
| Carotenoids (mg) | 672 |
609 |
318 |
682 |
209 |
381 |
| Superoxide dismutase (μg g–1) | 527.7 |
581.73 |
892.06 |
608.64 |
837.52 |
639.79 |
| Catalase (μg g–1) | 4166 |
3030 |
6250 |
1000 |
5000 |
6666 |
| Peroxidase (μg g–1) | 3676.4 |
2801.1 |
4310.3 |
2525.2 |
4385.9 |
2336.4 |
| Fresh weight (g) | 6.25 |
6.46 |
6.53 |
6.1 |
6.35 |
6.45 |
| Dry weight (g) | 2.2 |
1.9 |
2.1 |
2.1 |
1.8 |
2 |
| Ash contents (%) | 0.36 |
0.05 |
0.18 |
0.52 |
0.52 |
0.8 |
| Crude fibre (mg) | 21.333 |
18.567 |
10.5 |
23.467 |
23.167 |
23.2 |
| pH value | 7.1 |
6.66 |
6.7 |
6.5 |
5.7 |
6.6 |
Total antioxidant activity: In Calotropis procera, higher antioxidants concentration (61.8% DPPH inhibition) were recorded in leaves followed by (42.5%) roots. In Calotropis gigantea the higher antioxidant content (45.2%) was recorded for leaves as compared to roots (43.9%) and flowers (32.5%). In Calotropis procera flavonoid content was higher (2.803 mg/100 g) in the leaf extract followed by root extract (2.135 mg/100 g). In Calotropis gigantea flavonoid compounds were higher (2.2 mg/100 g) in the flower extract as compared to the leaf extract (2.194 mg/100 g).
Total carotenoids compounds: In Calotropis procera a higher content of total carotenoids (672 mg/100 g) was reported in leaves as compared to flowers (609 mg/100 g). In Calotropis gigantea the higher carotenoid content (682 mg/100 g) was observed in leaves as compared to the root extract (381 mg/100 g).
Enzymes studies: The enzymes studied the following important types were included. These were superoxide dismutase, catalase activity and peroxidase activity.
Superoxide dismutase (SOD) contents: In Calotropis procera higher values for superoxide dismutase enzyme were recorded for roots (892 μg g–1) while in Calotropis gigantea the higher (837 μg g–1) values were recorded in flower.
Catalase contents: In Calotropis procera higher catalase contents (6250 μg g–1) were recorded in root extract as compared to the leaves (4166 μg g–1) while lower catalase content (3030 μg g–1) in flowers. In Calotropis gigantea the catalase contents were higher (6666 μg g–1) in the root extract as compared to the flowers (5000 μg g–1), while the catalase contents (1000 μg g–1) were lower in the leaf extract.
Peroxidase (POX) contents: In Calotropis procera the peroxidase contents were higher (4310 μg g–1) in the roots as compared to leaves (3676 μg g–1) extract while lower peroxidase content (2801 μg g–1) were recorded in flower extract. In Calotropis gigantea the peroxidase content (4385 μg g–1) was noted in the flowers extract as compared to the leaves extract (2525 μg g–1), while the lower peroxidase contents (2336 μg g–1) were recorded in the roots extracts.
Proximate analysis: In Calotropis procera fresh leaves weight (6.25 g) and flowers fresh weight (6.46 g) and roots fresh weight (6.53 g) were observed. In Calotropis gigantea the fresh weight of leaves (6.1 g) and flowers (6.35 g) and roots fresh weight (6.45 g) were measured. The dry leaves’ weight in Calotropis procera was (2.2 g), flowers (1.9 g) and roots (2.1 g). In Calotropis gigantea the dry weight was 2.1 g for leaves, 1.8 g for flowers and 2.0 g for roots. The ash content in Calotropis procera was 0.36% in leaves, 0.05% in flowers and 0.18% in roots. In Calotropis gigantea ash content of leaves was (0.52%), flowers (0.52%) and roots (0.80%).
Crude fiber: In Calotropis procera the crude fiber content (21.3 mg) was higher in the leaf extract as compared to the flower extract (18.5 mg) while the lower contents (10.5 mg) of crude fiber was recorded in the root extract of Calotropis procera. In Calotropis gigantea the crude fiber was higher (23.4 mg) in the leaf extract as compared to flower (23.1 mg) and lower content (23 mg) of crfiberibre was recorded in the root extract of Calotropis gigantea.
pH: In Calotropis procera the pH was recorded in the leaf extract (7.1) while 6.6 were recorded in the flower extract. In Calotropis gigantea 6.6 pH value was noted in the root extract and a 5.7 pH value was recorded in the flower extract.
DISCUSSION
The phytochemical investigation is important as various significant elements are found in different plants. The present study was made for the screening of phytochemicals in Calotropis procera and Calotropis gigantea. Due to higher metabolite changes in enzymes and the bioactive compounds in plants, these compounds are helping to regulate plant growth. These phytochemicals have been described as preventing the growth of bacteria16. Flavonoids and phenols were higher in all parts of both species of Calotropis procera due to their environmental factors and biological effects these compounds also have valuable in common human diseases and plants and they play a major role in anti-cancer and other diseases. These compounds are naturally found in almost all plants17.
The various phytochemical screening (tannins, saponins, proteins, diterpenes, phytosterols flavonoids and phenols) were found in leaves and flowers of Calotropis gigantea14. The initial work has described the leaves extracts which contain bioactive molecules with a larger number such as alkaloids, saponins, tannins, flavonoids, proteins, phytosterols, diterpenes, etc. The presence of the compounds in these species demonstrates that they might have some therapeutic capability18. The phytochemical analysis by other scientists also confirmed the appearance of various phytochemicals19. Various parts of the plants have a huge perspective to cure several disorders and their use in several medicinal or herbal preparations10.
Phytochemically, the plants have been investigated for cardenolides, cardiac glycosides from the leaves, triterpenoids, anthocyanins from flowers and pentacyclic triterpenes, alkaloid, cardenolides phytosterols and triterpenoid saponins from the root Calotropis procera and Calotropis gigantea exhibit diversity in their chemical constituents and secondary metabolites even within a different part of the plant may be responsible for the difference in their antioxidant potential20.
Phenol is a necessary compound for the prevention of many diseases. The findings show that the total phenolic compound is remarkably higher in the flowers and leaves than in the root of both species. Comparatively, results show that the total phenolic compounds were higher in Calotropis procera as compared to Calotropis gigantea. The results are similar to Pietta21, who reported that the amount of total phenolic compounds varied widely with Calotropis procera specie the differences could be ecological collection time increasing population or environmental factors. According to Pietta21 phenolic compounds are the strongest chain-breaking antioxidants, their information is described in many plant-related studies and it describes various antioxidant activities of the plants in which phenolic compounds are high. As reported by Randhir and Shetty22 the higher phenolic compounds and improved antioxidant activity may be involved in the growth and development process.
The antioxidant activity was found by the 2,2-diphenyl picrylhydrazyl (DPPH) method for various parts (leaves, root, flower) of both species of (Calotropis procera and Calotropis gigantea). The higher activity of total antioxidants was observed in the leaves of both species. Results were similar to the findings of Wong et al.23, who suggested the variation in antioxidant activities of different medicinal plant species for their medicinal uses. Most plants contain compounds of flavonoids and tannins and some consist of phenolic compounds, which can also be responsible for antioxidant properties in various plants24.
The higher flavonoid compounds were present in the leaves of both species. The higher carotenoid contents were recorded in leaves of both species of Calotropis procera (672 mg/100 g) and Calotropis gigantea (682 mg/100 g).
The enzymes were analyzed superoxide dismutase, catalase and peroxide in both species. The results showed that in Calotropis procera the higher superoxide dismutase content (892 μg g–1) was recorded in root extract may be due to the application of growth regulators in different stages of micropropagation. Similar results were also reported by Kanmegne and Omokolo25. In Calotropis gigantea the higher content of superoxide dismutase (837 μg g–1) was recorded in flower extracts. Superoxide dismutase is one of the main and important enzymes which become concerned in cellular defense against reactive oxygen species in living organisms, hence, it is an important indicator of antioxidant capacity26.
The catalase enzyme indicated that the plant is herbal and beneficial for medicinal purposes. The catalase was observed higher (6250 μg g–1) in the root extract of Calotropis procera and lower (3030 μg g–1) was obtained in flowers. The higher activity may due to the endogenous H2O2 content and fall of superoxide dismutase activity in the cell. Catalase activity plays a role in the effective scavenging of H2O2 in cells under stressful conditions. Therefore, it can be concluded that the higher activity of catalase in field-grown plants could be due to the higher content of H2O2. In Calotropis gigantea the catalase content was higher in the (6666 μg g–1) extract of the root, while lower catalase content (1000 μg g–1) was in the leaf extract. The existence of the Catalase enzyme has shown that the purpose of plants is useful for therapeutic purposes of Calotropis procera and Calotropis gigantea might be due to the variation in plant species and chemical variations of changes in plants and their parts.
Peroxidase contents were also analyzed higher (4310 μg g–1) in the root extract of Calotropis procera may be due to the manifestation of protection against oxidative stress or signal induction required for growth and development and lower peroxidase contents (2801.1 μg g–1) were observed in flowers of Calotropis procera. In Calotropis gigantea the higher (4885 μg g–1) peroxidase was observed in flower extracts and lower peroxidase contents (2336 μg g–1) were obtained in root extracts of Calotropis gigantea. Comparatively, the results show that higher peroxidase contents were found in Calotropis procera as compared to Calotropis gigantea.
The proximate analysis was observed in several parts of both species of C. procera and C. gigantea. It reveals the dominant composition of fresh weight, dry weight, ash and crude fiber contents. The dry weight is essential for the primary net production rate.
The higher ash contents were found in Calotropis gigantea leaves. Fiber has a significant role in controlling many diseases such as diabetes, obesity and gastrointestinal tract diseases. The higher contents (21.3 mg) of crude fiber were found in Calotropis procera and it was higher due to more proteins and variations of phytochemicals. In Calotropis gigantea the higher (23.4 mg) was recorded in leaves and the lower (23.1 mg) was observed in flowers of Calotropis gigantea. The observation showed that there was a variety of changes in crude fiber concentrations in several parts of collected plants in the Haripur area. Crude fiber is one of the highest parameters observed, this means that it can become a source of nutritional fiber. Average raw fat content, which is generally the energy-saving form in living organisms. They are the main structural elements of biological layers such as phospholipids and sterols25.
The higher pH value ranges from 5.7 to 7.1. According to Laleh et al. 26, the pH values depend on the different compounds found in plants, the variations in abiotic conditions and the factors affecting pH, temperature and soil conditions.
The multifaceted biological characteristics of Calotropis procera and Calotropis gigantean make it a medicinally and socio-economically important species. The study evaluates its expanding global distribution, ecological significance, applications in traditional and advanced fields and infestation as an environmental weed. Also, it is an attempt to recognize the lesser-explored aspects and knowledge gaps in ongoing research.
Although pharmacological and industrial applications of these plants have received due attention, their general biological and ecological attributes have not been well-investigated. Evaluating the basic facets may improve its commercial utilization and pave the way for novel applications.
The current and potential spread of C. procera and C. gigantea are required to be mapped to carry out their time management or containment. The spread of both species can be effectively controlled in the invaded ranges via mechanical, chemical, or biological methods, followed by constant monitoring. Recognizing the plant as an important environmental weed can supplement its management programs at research, legislative, stakeholder and local levels. Also, promoting its utilization at commercial and non-commercial scales can be an economically viable or better to say, economically beneficial way of its management.
CONCLUSION
The Calotropis procera has more phytochemical constituents in leaves and roots as compared to Calotropis gigantea while the floral phytochemicals of Calotropis gigantea were higher as compared to flowers of Calotropis procera. The maximum phenolic compounds were recorded in the flowers of both species. The antioxidant activity was high in the leaves of both species. The flavonoid contents were maximum in the leaves of Calotropis procera and flower of Calotropis gigantea. The carotenoid contents were maximum in the leaves of both species. Superoxide dismutase concentration was maximum in the root of Calotropis procera. Catalase concentration was maximum in root of Calotropis gigantea. The peroxidase concentration was maximum in the flower of Calotropis gigantea. Maximum ash content was found in the root of Calotropis gigantea. Crude fiber value was maximum in leaves of Calotropis gigantea. The pH value was acidic in Calotropis gigantea extracts.
SIGNIFICANCE STATEMENT
This study discovered the phytochemical constituents of Calotropis procera and Calotropis gigantea. Both species have several different compounds and are therefore used for different human diseases like hepatoprotective, anticancer, antifertility, anti-inflammatory activities, skin disease, leukoderma, ulceration, boils, infectious diseases, swelling, tinea pedis, detach prickle from the body, immune diseases and Hansen's disease. Calotropis procera has more phytochemical constituents in leaves and roots as compared to Calotropis gigantea while the floral phytochemicals of Calotropis gigantea were found higher as compared to flowers of Calotropis procera. This study will help the researchers to uncover the critical areas of different phytochemical constituents and their uses in different diseases that many researchers were not able to explore.
ACKNOWLEDGMENT
I would like to acknowledge my supervising team and my co-authors.
REFERENCES
- Singh, S., S. Singh, R.M. Mishra and M.P. Shrivastava, 2014. Preliminary phytochemical screening of Calotropis gigantea leaf. Int. J. Sci. Res. Publ., 4.
- Sharma, P. and J.D. Sharma, 2000. In-vitro schizonticidal screening of Calotropis procera. Fitoterapia, 71: 77-79.
- Bahmani, M., H. Shirzad, M. Majlesi, N. Shahinfard and M. Rafieian-Kopaei, 2014. A review study on analgesic applications of Iranian medicinal plants. Asian Pac. J. Trop. Med., 7: S43-S53.
- Sadak, M.S., M.T. Abdelhamid and U. Schmidhalter, 2015. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb., 20: 141-152.
- Badouin, H., J. Gouzy, C.J. Grassa, F. Murat and S.E. Staton et al., 2017. The sunflower genome provides insights into oil metabolism, flowering and asterid evolution. Nature, 546: 148-152.
- Shobowale, O.O., N.J. Ogbulie, E.E. Itoandon, M.O. Oresegun and S.O.A. Olatope, 2013. Phytochemical and antimicrobial evaluation of aqueous and organic extracts of Calotropis procera ait leaf and latex. Niger. Food J., 31: 77-82.
- Faizul Haq, H. Ahmad, Rahat Ullah and Z. Iqbal, 2012. Species diversity and ethno botanical classes of the flora of Allai Valley District Battagram Pakistan. Int. J. Plant Res., 2: 111-123.
- Haq, F., H. Ahmad and M. Alam, 2011. Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. J. Med. Plants Res., 5: 39-48.
- Haq, F., S. Rehman, H. Ahmad, Z. Iqbal and R. Ullah, 2012. Elemental analysis of Paeonia emodi and Punica granatum by atomic absorption spectroscopy. Am. J. Biochem., 2: 47-50.
- Ghosh, T., M.K. Biswas, S. Chatterjee and P. Roy, 2018. In-vitro study on the hemolytic activity of different extracts of Indian medicinal plant Croton bonplandianum with phytochemical estimation: A new era in drug development. J. Drug Delivery Ther., 8: 155-160.
- Alnuqaydan, A.M. and B. Rah, 2019. Tamarix articulata (T. articulata)-An important halophytic medicinal plant with potential pharmacological properties. Curr. Pharm. Biotechnol., 20: 285-292.
- Lavanya, G.R., J. Srivastava and S.A. Ranade, 2008. Molecular assessment of genetic diversity in mung bean germplasm. J. Genet., 87: 65-74.
- Samarakoon, S.R., M.K. Ediriweera, L. Wijayabandara, N. Fernando and L. Tharmarajah et al., 2018. Isolation of cytotoxic triterpenes from the mangrove plant, Scyphiphora hydrophyllacea C.F.Gaertn (Rubiaceae). Trop. J. Pharm. Res., 17: 475-481.
- Ainsworth, E.A. and K.M. Gillespie, 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protocols, 2: 875-877.
- Kim, D.O., S.W. Jeong and C.Y. Lee, 2003. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem., 81: 321-326.
- Mayasari, D., D. Islami, E. Oktariani and P. Wulandari, 2023. Phytochemical, antioxidant and antibacterial evaluations of Ipomoea batatas L. from Riau, Sumatera Island, Indonesia. Trop. J. Nat. Prod. Res., 7: 2157-2162.
- Gyssels, G. and J. Poesen, 2003. The importance of plant root characteristics in controlling concentrated flow erosion rates. Earth Surf. Processes Landforms, 28: 371-384.
- Duan, Q., Z. Zhu, B. Wang and M. Chen, 2022. Recent progress on the salt tolerance mechanisms and application of tamarisk. Int. J. Mol. Sci., 23: 3325.
- Hossain, M.A. and Z. Ismail, 2013. Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus. Arabian J. Chem., 6: 295-298.
- Hassan, S., A. Atef, H.M. Ali, R. Alshamrani and A. Ramadan, 2022. First report of triterpenes pathway in Calotropis procera revealed to accumulate beta-amyrin. Saudi J. Biol. Sci., 29: 3647-3653.
- Pietta, P.G., 2000. Flavonoids as antioxidants. J. Nat. Prod., 63: 1035-1042.
- Randhir, R. and K. Shetty, 2004. Microwave-induced stimulation of L-DOPA, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochem., 39: 1775-1784.
- Wong, S.P., L.P. Leong and J.H.W. Koh, 2006. Antioxidant activities of aqueous extracts of selected plants. Food Chem., 99: 775-783.
- Aberoumand, A. and S.S. Deokule, 2008. Comparison of phenolic compounds of some edible plants of Iran and India. Pak. J. Nutr., 7: 582-585.
- Kanmegne, G. and N.D. Omokolo, 2003. Changes in phenol content and peroxidase activity during in vitro organogenesis in Xanthosoma sagittifolium L. Plant Growth Regul., 40: 53-57.
- Laleh, G.H., H. Frydoonfar, R. Heidary, R. Jameei and S. Zare, 2006. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak. J. Nutr., 5: 90-92.
How to Cite this paper?
APA-7 Style
Rehman,
N., Haq,
F., Faisal,
S. (2023). Phytochemical Analysis and Antioxidant Potential of Calotropis procera and Calotropis gigantea. Asian Journal of Biological Sciences, 16(3), 254-263. https://doi.org/10.3923/ajbs.2023.254.263
ACS Style
Rehman,
N.; Haq,
F.; Faisal,
S. Phytochemical Analysis and Antioxidant Potential of Calotropis procera and Calotropis gigantea. Asian J. Biol. Sci 2023, 16, 254-263. https://doi.org/10.3923/ajbs.2023.254.263
AMA Style
Rehman
N, Haq
F, Faisal
S. Phytochemical Analysis and Antioxidant Potential of Calotropis procera and Calotropis gigantea. Asian Journal of Biological Sciences. 2023; 16(3): 254-263. https://doi.org/10.3923/ajbs.2023.254.263
Chicago/Turabian Style
Rehman, Nadia, Faizul Haq, and Shah Faisal.
2023. "Phytochemical Analysis and Antioxidant Potential of Calotropis procera and Calotropis gigantea" Asian Journal of Biological Sciences 16, no. 3: 254-263. https://doi.org/10.3923/ajbs.2023.254.263

This work is licensed under a Creative Commons Attribution 4.0 International License.



