Quantitative Phytochemical Screening, Antioxidant and Aphrodisiac Potential Studies of Hydroethanolic Root Extract of Carissa spicatum L.
| Received 30 Nov, 2022 |
Accepted 29 Mar, 2023 |
Published 30 Jun, 2023 |
Background and Objective: Carissa spinarum is a plant used in West African countries such as Togo to manage erectile dysfunction. Despite this usage, there are no scientific proof of its aphrodisiac potential. This work was then, carried out to evaluate the aphrodisiac potential of Carissa spinarum roots hydro-alcoholic extract associated with its quantitative phytochemical composition and its antioxidant activity. Materials and Methods: Prior to the aphrodisiac potential study, the quantitative phytochemical screening and the antioxidant activity of the extract were assessed. The quantitative phytochemical screening was done to determine the amount of tannin, total phenols, flavonoids and cardiac glycosides in the extract. The antioxidant properties were performed through total antioxidant capacity (TAC), reducing power and, free radicals 1,1-diphenyl-2-picrylhydrazyl (DPPH) eliminating activity. The aphrodisiac potential of the extract was evaluated by studying its effect (75 and 150 mg kg‾1) on sexual behavior (number of sexual rises and anogenital sniffing as well as their respective latency) in male ICR mice compared to sildenafil (5 mg kg‾1). Results: The hydroethanolic root extract of Carissa spinarum contained 23.21±1.42 mg Eq AG/g of extract of total phenol, 14.97±0.30 mg Eq of rutin/g of extract of flavonoids, 7.09±1.42 mg of Eq AG/g of extract of tannins and 7.84±0.42 mg d’Eq of digoxin/g of extract of cardiac glycosides. The extract produced antioxidant activity and significantly altered sexual behavior parameters, especially at the dose of 150 mg kg‾1. Thus, the number of sexual mounting and anogenital sniffing was significantly increased (p<0.0001). Inversely, their respective latency decreased significantly (p<0.0001). Conclusion: It emerges from this study, that the hydroethanolic extract of Carissa spinarum roots has antioxidant properties and aphrodisiac activities.
| Copyright © 2023 Mawubédjro et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Erectile Dysfunction (ED) is defined as an inability to produce a sufficient erection to ensure sexual intercourse1,2. It has become a real social problem around the world and has an ever-increasing incidence, from 152 million people in 1995, nearly 322 million people are believed to be affected by researchers 20253-5. Its occurrence is due to some physiological factors such as age or some pathological situations such as cardiovascular diseases, diabetes6 and oxidative stress7,8. Regarding oxidative stress, interactions between free radicals and nitric oxide (NO) can lead to erectile dysfunction9. To manage erectile dysfunction several remedies are proposed. Among them, substances that increase sexual desire, called aphrodisiacs are used to enhance the fertility rate. In conventional medicine, molecules like phosphodiesterase 5 inhibitors (sildenafil) are, therefore, used. Other remedies are also used such as penile implants, or even hormones10. However, in developing countries, traditional medicine based on the use of medicinal plants remains the most popular approach for the management of erectile dysfunction11. Thus, several plants are used for this purpose, such as Garcinia cola12, Mondia whitei13, Withania somnifera, Asphaltum, Tribulus terrestris, Asparagus racemosus and Sida cordifolia5. Carissa spinarum is one of the plants used in the West African sub-region, especially in Togo to manage erectile dysfunction14,15. Despite its widespread use for this purpose, little scientific data exists to confirm its aphrodisiac effect. This study was then conducted to assess the aphrodisiac potential of the hydroethanolic extract of C. spinarum roots in association with its quantitative phytochemical composition and antioxidant properties, to establish a scientific basis for its use in traditional medicine as an aphrodisiac.
MATERIALS AND METHODS
Study area: This study was carried out from September, 2021 to February, 2022 at the University of Lomé, Togo in the Laboratory of the Animal Physiology and Pharmacology Department and in the Laboratory of the Pharmaceuticals Sciences Department.
Materials
Collection and extraction of plant material: The methodology used for plant collection and extraction was the same as used in previous studies by Dossou-Yovo et al.15. Thus, samples composed of Carissa spinarum roots were collected at Noépé (6°17'38"N and 1°1'19"E, Togo). They were identified at the Botany and Ecology Department of the University of Lomé and a voucher was kept at the herbarium under N°TOGO15579. After being cleaned and dried under climatization (20°C) in the laboratory of the Animal Physiology and Pharmacology department of the University of Lomé, roots were dried, pulverized and reduce into powder. The powder was soaked in mixed ethanol-water (50-50) for 72 hrs under automatic agitation on an orbital shaker (IKA KS 501 orbital digital platform shaker). The filtrate obtained after a double filtration with cotton and whatman paper was evaporated at 45°C using a rotating evaporator (IKA RV 10 Digital).
Animals: For the aphrodisiac test, 30, Inactivated Embryonic Fibroblasts (ICR) mice (Mus musculus) of both sexes, provided by the Animal Physiology and Pharmacology Department, were used. They were acclimatized at least 1 week prior to the manipulations and fed with standard diets and water ad libitum. Mice, before and during the study were kept at 22±2°C. The relative humidity level was 40% and the photoperiod was 12 hrs light and 12 hrs darkness. Animal care and handling conformed to accepted guidelines15.
Ethical consideration: Ethical approval was obtained from the institutional Ethical Committee for Teaching and Research under the number: Ref No. CNCB-CEER 2801/2010.
Experimental design
Quantitative phytochemical screening and antioxidant activities of Carissa spinarum hydroethanolic roots extract
Quantitative phytochemical screening: Total phenols were dosed by the method described by Maksimovic et al.16. Flavonoids and tannins were dosed according to Povi et al.17 method. Additionally, cardiac glycosides were also dosed by Tofighi et al.18 method.
Total phenols and tannins dosage: The principle of the determination of total phenols lies in the oxidation of phenolic compounds by the Folin-Ciocalteu reagent consisting of a mixture of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O40). The reagent is reduced during the oxidation of phenols in a blue mixture of tungsten oxide (W40O23) and molybdenum (Mo8O23) which absorbs at about 750 nm. The fixation of the tannins by polyvinyl polypyrrolidone (PVPP) during a second dosage makes it possible to deduce their quantity from the total quantity of phenols.
The test was carried out by adding 200 μL of 10% Folin-Ciocalteu reagent to 200 μL of extract (1 mg mL–1 dissolved in 95% methanol) or to 200 μL of supernatant of the extract solution fixed by the PVPP (tannins fixed beforehand by adding 500 of to 500 μL of 95% methanol containing 10 mg of PVPP). After incubation at 28±2°C for 15 min, 800 μL of sodium carbonate solution (700 mM) was added. The absorbance was read at 735 nm against the blank. Gallic acid (0, 5, 25, 50 and 100 μg mL–1) was used as a standard to plot the calibration curve19.
The amount of total phenol and that of tannin were expressed in mg of gallic acid equivalent/g of extract. Three trials were performed for each sample.
Flavonoids dosage: To determine the flavonoids quantity, 2 mL of aluminum chloride (20 mg mL–1) and 6 mL of sodium acetate (50 mg mL–1) were added to 2 mL of the extract or the range of rutin (5, 25, 50, 75 and 100 μg mL–1). The optical density was read at 440 nm against a blank (2 mL of ethanol) after 150 min of incubation. Rutin optical densities were used to plot the calibration curve.
The amount of flavonoid was expressed in mg of rutin equivalent/g of extract. Three trials were performed for each sample.
Determination of cardiotonic glycosides: To 1 mL of the defatted extract was added 10 mL of Baljet’s reagent. After a one-hour of incubation, 20 mL of distilled water was added. The absorbance of the mixture was read with a spectrophotometer at 495 nm. Cardiotonic glycosides quantity was determined from a calibration curve of the digoxin range (10, 30, 50, 70 and 100 μg mL–1). Results were expressed in mg of digoxin equivalent per gram of dry extract (mg d’Eq/g). Three trials were performed for each sample.
Antioxidant activity: The antioxidant activity of Carissa spinarum hydro-alcoholic root extract was determined by evaluating its total antioxidant capacity, reducing power and anti-free radical power (DPPH).
Total antioxidant capacity: The total antioxidant activity of the extract was evaluated according to the method of Kumaran and Karunakaran20, based on the use of phosphomolybdenum. To 0.3 mL of extract (1 mg mL–1) 3 mL of reagent was added. The reagent composition was: 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. Samples were vortexed, were and incubated at 95°C for 90 min. Ascorbic acid was used as a standard in a range of 25, 50, 75, 100, 150, 200 and 250 μg mL–1. After incubation samples were cooled at room temperature and the absorbance of the solution was read at 695 nm against blank. A standard curve of ascorbic acid was plotted. Antioxidant activity was expressed as mg of the equivalent of ascorbic acid per gram of extract (mg Eq AA/g). The number of samples was 3 for each concentration.
Reducing power: This test is based on the reduction capacity of Fe3+ (ferric ion) to Fe2+ (ferrous ion). By accepting an electron, Fe3+ then becomes Fe2+ and a green coloration is observed which is all the more intense the higher the reducing power21.
Different concentrations of extract (0.5 mL) were then mixed with 1.25 mL of phosphate buffer (pH = 6.6) and 1% potassium ferricyanide. The reaction mixture was incubated in a water bath at 50°C for 20 min. After cooling, 1.25 mL of 10% trichloroacetic acid was added to stop the reaction. The reaction mixture was then centrifuged for 10 min at 3000 rpm. After that 1.25 mL of the top layer of the solution was mixed with 1.25 mL of distilled water and 0.25 mL of 0.1% ferric chloride solution. Ascorbic acid served as the standard. The standard and extract ranges were at concentrations of 25, 50, 100, 200 and 400 μg mL–1. The absorbance was read at 700 nm and curves were plotted using optical density values. The number of samples was 3 for each concentration.
DPPH test: Free radicals 1,1-diphenyl-2-picrylhydrazyl (DPPH) eliminating activity was evaluated by the method of Kumaran and Karunakaran20 with slight modifications, in particular in terms of volumes of reagents used. Thus 1.5 mL of DPPH solution (100 μM) and 0.25 mL of methanol or 0.25 mL of methanolic extract at different concentrations (31.25, 62.5, 125, 250 and 500 μg mL–1) have been mixed up. The absorbance was read at 517 nm after 30 min of incubation. Ascorbic acid at different concentrations was used as a reference (12.5, 25, 50, 100 and 200 μg mL–1). The percentage of anti-free radical activity was calculated from the following formula:
where, A0 is the absorbance of the control and A1 is the absorbance of the extract or standard. These percentages were used to draw regression curves. The inhibitory concentration 50 (IC50) was then determined for the ascorbic acid and extracted from these regression curves. The number of samples was 3 for each concentration of the extract or ascorbic acid.
Aphrodisiac test: Sexual behavior parameters such as sexual mount and anogenital sniffing frequency and their latency were evaluated in male ICR mice (28.60±1.29 g). The sexual mount is defined as the male assuming the copulatory position but failing to achieve intromission and anogenital sniffing is defined as occasions when the male sniffs the genital part of the female22. So, the parameters evaluated are defined as follows:
| • | Sexual mount frequency is the number of mounts that occurred when the female is introduced in the cage | |
| • | Anogenital sniffing frequency is the number of anogenital sniffing that occurred when the female is introduced into the cage | |
| • | Sexual mount latency is the time since female introduction in the cage and the first mount | |
| • | Anogenital sniffing latency is the time since female introduction in the cage and the first anogenital sniffing |
Animal selection: Before tests, the mice were observed for sexual behavior twice a day for 1 week. Only those who presented sexual behavior were selected for the study.
Protocol: The aphrodisiac potential of Carissa spinarum hydro-alcoholic root extract was evaluated following the protocol described by Muralidharan and Gejalakshmi22. Nevertheless, little modifications were made mainly on the observation period duration and the number of contacts between males and females.
Twenty-four male ICR mice were grouped into 4 batches of 6 animals each:
| • | Negative control group which received distilled water | |
| • | Positive control group which received sildenafil at 5 mg kg–1 | |
| • | And two treated groups which received the extract respectively at 75 and 150 mg kg–1 |
The solutions were administered orally for 6 days at 5 mL kg–1 and sexual parameters were recorded at day 0, day 3 and day 6. Thus, 30 min after solution administration, male mice were put individually in the cage for 15 min before female’s introduction (one female per male). Sexual parameters were then recorded for 15 minutes. After each contact of 15 min, females were retired from the cage for 15 min before another reintroduction. Four contacts between males and females were achieved during the experimentation.
Statistical analysis: Results of aphrodisiac tests were analyzed by using One-way ANOVA by GraphPad 6.02 software (GraphPad Software, Inc., USA) The difference between the two groups was determined by using Tukey’s test. The significance level was set at p<0.05.
RESULTS
Quantitative phytochemical screening and antioxidant activities of Carissa spinarum hydroethanolic roots extract
Quantitative phytochemical screening: Total phenols, tannins, flavonoids and cardiotonic glycoside quantities in the extract are noticed in Table 1. Then, the extract component was 23.21±1.42 mg Eq of gallic acid/g of extract of total phenols, 7.09±1.42 mg d’Eg of gallic acid/g of extract of tannins, 14.97±0.30 mg d’Eq of rutin/g of extract of flavonoids and 7.84±0.42 mg d’Eq of digoxin/g of extract of cardiac glycosides.
Antioxidant activities
Total antioxidant capacity: The optical densities read allow us to plot a standard curve of ascorbic acid. The total antioxidant capacity was 50.04±0.61 mg Eq of ascorbic acid/g of extract.
Reducing power: The reducing power of the extract was concentrations-depending but was lower than ascorbic acid reducing power (Fig. 1).
DPPH: The hydroethanolic extract of Carissa spinarum roots IC50 was 0.103±0.020 mg mL–1 and the ascorbic acid one was 0.0780±0.0005 mg mL–1.
| Table 1: | Quantitative phytochemical screening | |||
| Chemical group | Result |
| Total phenols (mg Eq of gallic acid/g of extract) | 23.21±1.42 |
| Tannins (mg d’Eg of gallic acid/g of extract) | 7.090±1.42 |
| Flavonoids (mg d’Eq of rutin/g of extract) | 14.97±0.30 |
| Cardiac glycosides (mg d’Eq of digoxin/g of extract) | 7.840±0.42 |
| Each value is a mean±standard error on the mean, n = 3, Eq: Equivalent, mg: Milligram and g: Gram | |

|
|
Aphrodisiac tests
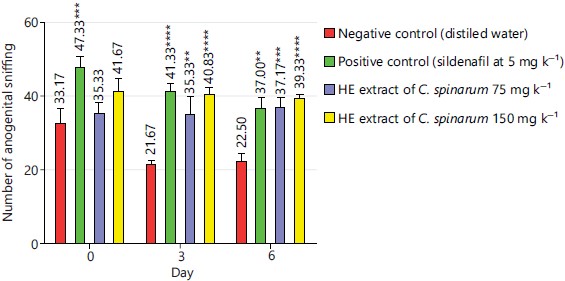
Anogenital sniffing frequency: On day 0, only the sildenafil at 5 mg kg–1 induced a significant increase of the anogenital sniffing frequency (p<0.001) when compared to the negative control group. Thus, anogenital sniffing was 47.83±6.45 in the sildenafil batch and 33.17±8.33 in the negative control batch. From day 3 to 6, the sildenafil at 5 mg kg–1 as well as the extract at different doses increased significantly the anogenital sniffing frequency (p<0.05) when compared to the negative control group (Fig. 2). So, on day 6, anogenital sniffing in the extract batch at 150 mg kg–1 was 39.33±2.84 against 37.00±3.15 in the sildenafil batch and 22.50±4.89 in the negative control batch.
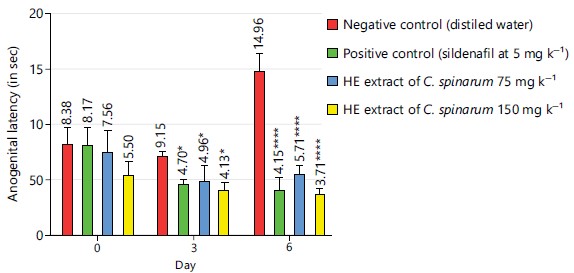
Anogenital sniffing latency: Concerning the anogenital sniffing latency, on day 0, no significant difference was observed (p>0.05) when compared to the negative control group. From day 3 to 6, the sildenafil at 5 mg kg–1 and the extract at different doses, both decreased significantly the anogenital sniffing latency (p<0.05) when compared to the negative control group. The most important decrease was observed in the extract batch at 150 mg kg–1, where it was 3.71 sec±0.5 against 14.96 sec±1.51 in the negative control group (Fig. 3).
Sexual mount frequency: On day 0, a significant increase was observed (p<0.001) in the sildenafil group at 5 mg kg–1 and in extract groups (75 and 150 mg kg–1) when compared to the negative control group. From day 3 to 6, the increase was maintained when comparing a negative control group to other groups. The mounting frequency on days 0 and 3 was more significant in the sildenafil (5 mg kg–1) batch but on day 6 it was 15.00±2.40 in the sildenafil group while in the extract batch at 150 mg kg–1, it was 15.8±2.90 (Fig. 4).
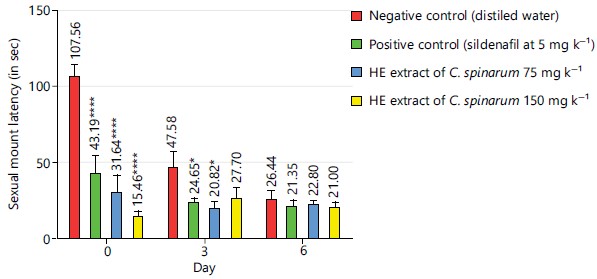
Sexual mount latency: Results obtained for sexual mount latency on day 0, showed a significant decrease (p<0.001) in the sildenafil (5 mg kg–1) batch and in extract (75 and 150 mg kg–1) groups when compared to the negative control group. On day 3, the sildenafil at 5 mg kg–1 and the extract at 75 mg kg–1, decreased significantly the sexual mount latency (p<0.05) when compared to the negative control group. On day 6, no significant difference was observed when comparing negative control group with others (Fig. 5).
|

|
|
DISCUSSION
In this study, the quantitative phytochemical screening allowed us to quantify some phytochemical groups mainly, total phenols, flavonoids, tannins and especially cardiotonic glycosides present in the extract. Thus, the quantity of total phenol was 23.21±1.42 mg Eq AG/g of extract, that of flavonoids 14.97±0.30 mg Eq of rutin/g of extract and that of tannins 7.09±1.42 mg of Eq AG/g of extract. These results concerning the flavonoid content are similar to those of Yadang et al.21, who found a flavonoid content of 14.84±0.013 mg of rutin Eq/g of extract during their work on the leaves of Carissa spinarum, a plant used traditionally as aphrodisiac14,23. The total phenol and tannin contents are lower than those found during this work. This difference could be explained by the part of the plant studied. Similarly, other work on the roots of Carissa spinarum has revealed the presence in similar amount of flavonoid (13.35±0.57 mg of rutin Eq/g of extract) and total phenol (35.44±0.13 mg of Eq AG/g of extract) in the hydroalcoholic root extract of Carissa spinarum14. Among these different chemical groups characterized in previous study Dossou-Yovo23 and measured in the hydroethanolic extract of the roots of Carissa spinarum, some, such as flavonoids, are said to have antioxidant activity24.
Indeed, oxidative stress is considered to be one of the factors in the onset of erectile dysfunction7,8. In addition, studies on animal models have evidenced the existence of a link between the occurrence of erectile dysfunction and free radicals, especially in people with diabetes25,26. This could be explained by interactions between free radicals and nitric oxide which could lead to erectile dysfunction9. Because of this link, antioxidant tests have been carried out to assess the antioxidant activity of the plant. These tests confirmed the antioxidant properties of the hydroethanolic extract of the roots of Carissa spinarum as indicated by previous research on the plant21.
The hydroethanolic extract of the roots of Carissa spinarum exhibited a total antioxidant capacity of 50.04±0.61 mg Eq AA/g. This result confirmed the presence of antioxidant compounds such as phenolic compounds identified during phytochemical screening, in the extract24. One of the main mechanisms of action of phenolic antioxidant compounds is their ability to donate electrons27. Thus, during reducing power tests, Fe3+ ions gain an electron to become Fe2+ ions. The hydroethanolic extract of the roots of Carissa spinarum exhibited a lower reducing power than that of vitamin C which was used as a reference. However, the hydroethanolic extract of the roots of Carissa spinarum would therefore have the ability to reduce ferric ions to ferrous ions by donating electrons. Results are similar to those of previous work which underlined the reducing power of extracts from the roots and leaves of Carissa spinarum21,28.
One of the hallmarks of antioxidant compounds is anti-free radical activity. Its evaluation is based on the discoloration of the DPPH radical. This discoloration is due to the ability of antioxidant molecules to donate hydrogen atoms29,30. The results obtained indicated that the IC50 for the extract was 0.103±0.02 and 0.078±0.0003 mg mL–1 for ascorbic acid. The IC50 obtained in this study is close to that of Yaovi-Gameli et al.14, who found an IC50 of 0.096±0.001 mg mL–1 for hydro-alcoholic extracts from the roots of Carissa spinarum.
Phenolic compounds such as tannins, phenols and flavonoids would be the main phytochemical groups responsible for the antioxidant activity of the plant30, especially since previous results confirmed the presence of these phenolic compounds in the hydroethanolic extract of the roots of Carissa spinarum23. Their anti-radical power or their ability to scavenge free radicals can be explained by the presence of the phenolic hydroxyl group28,31. Previous work carried out on both leaves and roots of Carissa spinarum also attributed its antioxidant properties to the phenolic compounds it contains21. This antioxidant activity could be beneficial in inhibiting the oxidative processes involved in the occurrence and installation of certain pathologies such as erectile dysfunction7,8.
The assessment of the aphrodisiac potential of the extract was based on the study of some sexual behavior parameters such as the number and latency of sexual mounting32,33 and the number and latency of anogenital sniffing22. In fact, parameters such as the number and latency of sexual mounting and anogenital sniffing are parameters usually evaluated to determine the aphrodisiac properties of plants22. The results obtained showed that the hydroethanolic extract of the roots of Carissa spinarum significantly increased (p<0.001) the number of sniffing, especially at a dose of 150 mg kg–1 at which an effect similar to that of sildenafil was observed on day 3 and day 6. Likewise, the extract at 150 mg kg–1 significantly increased the number of sexual mountings similarly to sildenafil on day 3 (p<0.001) and day 6 (p<0.0001). This increase in the number of sexual mounts and anogenital sniffing would indicate a stimulating effect of the extract on sexual desire and copulatory activity33. The latencies of sniffing and mounting were significantly reduced. This reduction in the sniffing latency had the same significance (p<0.0001) in the treated and positive control batches on day 6. As for the mounting latency, sniffing latency decreased in all the batches as a function of time without showing any significant variation on day 6 (p>0.05) unlike on day 0 and day 3 where significant differences were observed. In fact, latency periods are inversely proportional to sexual motivation33. They are indicators of the hesitation time of males following the presentation of the female34. The reduction in latencies would therefore, reflect a stimulating effect of C. spinarum hydroethanolic extract of the roots on sexual motivation. The absence of significance on day 6 for latency periods between groups may be due to the accustomedness phenomenon. Similar studies on aphrodisiac plants such as Spathodea campanulata33, Garcinia cola12 and Desmodium velutinum11, have led to similar observations regarding the sexual parameters evaluated. These results would therefore, indicate that the hydroethanolic extract of the roots of C. spinarum would have a pro-sexual effect. This aphrodisiac potential of the plant is thought to be due to phytochemical groups such as saponins and flavonoids it contains34,35. Thus, saponins by their steroidal nature could promote the formation of androgens, like testosterone33. In addition, they would have a relaxing effect on vascular smooth muscle by stimulating the release of No36. This relaxation is essential for an erection. Flavonoids, by their antioxidant activity, can contribute to explaining the aphrodisiac properties of the plant as antioxidant activity is helpful in inhibiting the occurrence and installation of erectile dysfunction7,8,24. These results could justify the use of C. spinarum roots in the management of erectile dysfunction. As shown in this study, Carissa spinarum has aphrodisiac properties. Thus, it can be used for erectile dysfunction management. Nevertheless, studies must be conducted in future to deepen the aphrodisiac activities of the plant and its safety, mainly for the cardiovascular system.
CONCLUSION
In this study, the quantitative phytochemical screening, antioxidant activity and the aphrodisiac potential of the hydroethanolic root extract of C. spinarum were carried out. The quantitative phytochemical screening allowed us to quantify some compounds of the extract such as total phenols, tannins, flavonoids and cardiac glycosides. The hydroethanolic extract of C. spinarum roots showed antioxidant activity through its total antioxidant capacity, its reducing power and its capacity to scavenge free radicals. Sexual behavior parameters evaluated in this study indicated that the extract has aphrodisiac potential. This aphrodisiac potential associated with the antioxidant activity can explain the traditional use of Carissa spinarum roots to manage erectile dysfunction. Nevertheless, future research will be carried out to explore more sexual parameters to enhance scientific data available on the plant used as an aphrodisiac.
SIGNIFICANCE STATEMENT
Many plants are used for erectile dysfunction through the world without any scientific proof. In Togo, one of the plants used is Carissa spinarum. The aim of this study is to establish a scientific support of the use of Carissa spinarum as an aphrodisiac plant. This is the first study conducted in Togo to give scientific support to plants or molecules used as aphrodisiacs in the country. The results have shown that Carissa spinarum hydroethanolic root extract has aphrodisiac properties as it enhances some sexual behavior parameters. This study is therefore a base for the researchers for the evaluation of the effectiveness of plants traditionally used for erectile dysfunction management.
ACKNOWLEDGMENTS
We thank the TWAS (The World Academy of Science) for its free funding (Grant No. 19-242 RG/BIO/AF/ AC_G) during the research work for its support to the Department of Pharmacy of th University of Lomé, Togo where some of the experimentations were conducted on materials and apparatus provided through this grant.
REFERENCES
- McVary, K.T., 2007. Erectile dysfunction. New Engl. J. Med., 357: 2472-2481.
- Ajao, A.A., N.P. Sibiya and A.N. Moteetee, 2019. Sexual prowess from nature: A systematic review of medicinal plants used as aphrodisiacs and sexual dysfunction in Sub-Saharan Africa. South Afr. J. Bot., 122: 342-359.
- Meuleman, E.J.H., 2003. Review of tadalafil in the treatment of erectile dysfunction. Expert Opin. Pharmacother., 4: 2049-2056.
- Oladiji, F., O.O. Kayode and D.B. Parakoyi, 2013. Influence of socio-demographic characteristics on prevalence of erectile dysfunction in Nigeria. Int. J. Impotence Res., 25: 18-23.
- Tiwari, R., S. Choudhary and G. Gaba, 2018. Pharmacological and toxicological evaluation of herbal extracts in rat model of erectile dysfunction. Int. J. Herb. Med., 6: 9-16.
- Melman, A. and J.C. Gingell, 1999. The epidemiology and pathophysiology of erectile dysfunction. J. Urol., 161: 5-11.
- Codoñer-Franch, P., V. Valls-Bellés, A. Arilla-Codoñer and E. Alonso-Iglesias, 2011. Oxidant mechanisms in childhood obesity: The link between inflammation and oxidative stress. Transl. Res., 158: 369-384.
- Sarr, S.O., A.D. Fall, R. Gueye, A. Diop and K. Diatta et al., 2015. Study of the antioxidant activity of extracts from the leaves of Vitex doniana (Verbenacea) [In French]. Int. J. Biol. Chem. Sci., 9: 1263-1269.
- Minhas, S., J.Y. Jeremy, R.W.A. Jones, D. Ralph, R.W. Rees and R.A. Persad, 2002. Oxygen free radicals and the penis. Expert Opin. Pharmacother., 3: 889-897.
- Hess, C. and A. Boehmer, 2020. Toxicology of natural and synthetic aphrodisiacs. Rechtsmedizin, 30: 15-30.
- Mafo, A.R., O.W. Otaru, S.F. Ajuma, M.T. Boniface and I.P. Adejoh, 2020. Evaluation of the fertility activity of aqueous leaf extract of Desmodium velutinum in male Wistar rats. Int. J. Adv. Res. Biol. Sci., 7: 1-8.
- Ralebona, N., C.R. Sewani-Rusike and B.N. Nkeh-Chungag, 2012. Effects of ethanolic extract of Garcinia kola on sexual behaviour and sperm parameters in male Wistar rats. Afr. J. Pharm. Pharmacol., 6: 1077-1082.
- Joseph, O., T.J. Kihdze, B. Katusiime, L. Imanirampa, P. Waako, F. Bajunirwe and A.A. Ganafa, 2015. Toxicity of four herbs used in erectile dysfunction: Mondia whiteii, Cola acuminate, Urtica massaica, and Tarenna graveolensin male rats. Afr. J. Pharm. Pharmacol., 9: 756-763.
- Yaovi-Gameli, A., K. Koffi, E. Komlavi, A. Amegnona, T. Koffi and G. Messanvi, 2018. An ethnobotanical survey of medicinal plants used in the preparation of “Atikédi”: Local alcoholic beverages commonly consumed in Lomé Togo. Eur. Sci. J., 14: 1-16.
- Dossou-Yovo, K.M., A. Diallo, P. Lawson-Evi, Y.T. Kantati, T. Darré, B. Bakoma and K. Eklu-Gadégbéku, 2021. A 90-day oral toxicity of hydroethanolic root extract of Carissa spinarum in Wistar rats. J. Toxicol., 2021: 5570206.
- Maksimović, Z., Đ. Malenčić and N. Kovačević, 2005. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol., 96: 873-877.
- Povi, L.E., B. Batomayena, T.A. Hode, E.G. Kwashie, A. Kodjo and G. Messanvi, 2015. Phytochemical screening, antioxidant and hypoglycemic activity of Coccoloba uvifera leaves and Waltheria indica roots extracts. Int. J. Pharm. Pharm. Sci., 7: 279-283.
- Tofighi, Z., G. Saeidi, A. Hadjiakhoondi and N. Yassa, 2016. Determination of cardiac glycosides and total phenols in different generations of Securigera securidaca suspension culture. Res. J. Pharmacogn., 3: 25-31.
- Atchou K., P. Lawson-Evi, K. Metowogo, B. Bakoma, K. Eklu-Gadegbeku, K. AkliKokou and M. Gbeassor, 2020. Antihyperglycaemic and antioxidant activities of Crataeva adansonii DC. ssp. Adansonii leaves extract on ICR mice. J. Drug Delivery Ther., 10: 30-38.
- Kumaran, A. and R.J. Karunakaran, 2007. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci. Technol., 40: 344-352.
- Yadang, S.A.F., G.T. Sotoing, K.S.N. Zouakeu, M.A. Khan, G.A. Agbor, N. Ur-Rahman and E.N. Bum, 2019. Quantification of bioactive compounds and evaluation of the antioxidant activity of Carissa edulis Valh (apocynaceae) leaves. Sci. World J. 2019: 7549620.
- Muralidharan, P. and S. Gejalakshmi, 2017. Evaluation of erogenic activity of hydroalcoholic extract of Lemanea fluviatilis in rats. World J. Pharm. Res., 6: 663-674.
- Dossou-Yovo, K.M., A. Diallo, P. Lawson-Evi, T. Darré, B. Bakoma and K. Eklu-Gadégbéku, 2021. Cytotoxicity, acute, and subacute study of hydroalcoholic root extract of Carissa spinarum L. on Wistar rats. J. Med. Food, 24: 756-761.
- Nijveldt, R.J., E. van Nood, D.E.C. van Hoorn, P.G. Boelens, K. van Norren and P.A.M. van Leeuwen, 2001. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr., 74: 418-425.
- Kim, J.H., H. Kim, Y.H. Kim, W.S. Chung, J.K. Suh and S.J. Kim, 2013. Antioxidant effect of captopril and enalapril on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Thorac. Cardiovasc. Surg., 46: 14-21.
- Castela, A., P. Gomes, V.F. Domingues, P. Paíga, R. Costa, P. Vendeira and C. Costa, 2015. Role of oxidative stress-induced systemic and cavernosal molecular alterations in the progression of diabetic erectile dysfunction. J. Diabetes, 7: 393-401.
- Machu, L., L. Misurcova, J.V. Ambrozova, J. Orsavova, J. Mlcek, J. Sochor and T. Jurikova, 2015. Phenolic content and antioxidant capacity in algal food products. Molecules, 20: 1118-1133.
- Woode, E., C. Ansah, G.K. Ainooson, W.M. Abotsi, A.Y. Mensah and M. Duweijua, 2007. Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (Forsk.) Vahl (Apocynaceae). J. Sci. Technol., 27: 6-15.
- Aksoy, L., E. Kolay, Y. Agilonu, Z. Aslan and M. Kargioglu, 2013. Free radical scavenging activity, total phenolic content, total antioxidant status and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci., 20: 235-239.
- Dai, J. and R.J. Mumper, 2010. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15: 7313-7352.
- Wetchakul, P., P. Chonsut, C. Punsawad and S. Sanpinit, 2022. LC-QTOF-MS characterization, antioxidant activity, and in vitro toxicity of medicinal plants from the Tri-Than-Thip remedy. Evidence-Based Complementary Altern. Med., 2022: 4477003.
- Dhawan, K., S. Kumar and A. Sharma, 2003. Aphrodisiac activity of methanol extract of leaves of Passiflora incarnate Linn. in mice. Phytotheray Res., 17: 401-403.
- Clovis, T., I. Yaya, N. Nga, D.S. Didier and M.M. Emmanuel 2019. Evaluation of aphrodisiac properties of the aqueous extract of the trunk barks of Spathodea campanulata P. Beauv. (Bignoniaceae) on albino rats (Rattus norvegicus). J. Med. Plants Res., 13: 480-486.
- Yakubu, M.T., O.S. Awotunde, O.T. Ajiboye, A.T. Oladiji and M.A. Akanji, 2011. Pro-sexual effects of aqueous extracts of Massularia acuminata root in male Wistar rats. Andrologia, 43: 334-340.
- Drewes, S.E., J. George and F. Khan, 2003. Recent findings on natural products with erectile-dysfunction activity. Phytochemistry, 62: 1019-1025.
- Fikriah, I., S. Ismail and K. Kosala, 2021. In vitro evaluation of the vasodilatory activity of ethanol extracts of Eleutherine bulbosa bulbs and leaves. J. Appl. Pharm. Sci., 11: 135-140.
How to Cite this paper?
APA-7 Style
Mawubédjro,
D.K., Diallo,
A., Lawson-Evi,
P., Kantati,
Y.T., Atchou,
K., Kueviakoé,
D.I., Assih,
M., Sanvee,
S., Badjabaïsi,
E. (2023). Quantitative Phytochemical Screening, Antioxidant and Aphrodisiac Potential Studies of Hydroethanolic Root Extract of Carissa spicatum L.. Asian Journal of Biological Sciences, 16(2), 155-166. https://doi.org/10.3923/ajbs.2023.155.166
ACS Style
Mawubédjro,
D.K.; Diallo,
A.; Lawson-Evi,
P.; Kantati,
Y.T.; Atchou,
K.; Kueviakoé,
D.I.; Assih,
M.; Sanvee,
S.; Badjabaïsi,
E. Quantitative Phytochemical Screening, Antioxidant and Aphrodisiac Potential Studies of Hydroethanolic Root Extract of Carissa spicatum L.. Asian J. Biol. Sci 2023, 16, 155-166. https://doi.org/10.3923/ajbs.2023.155.166
AMA Style
Mawubédjro
DK, Diallo
A, Lawson-Evi
P, Kantati
YT, Atchou
K, Kueviakoé
DI, Assih
M, Sanvee
S, Badjabaïsi
E. Quantitative Phytochemical Screening, Antioxidant and Aphrodisiac Potential Studies of Hydroethanolic Root Extract of Carissa spicatum L.. Asian Journal of Biological Sciences. 2023; 16(2): 155-166. https://doi.org/10.3923/ajbs.2023.155.166
Chicago/Turabian Style
Mawubédjro, Dossou-Yovo, Komlan, Aboudoulatif Diallo, Povi Lawson-Evi, Yendubé Touguelighan Kantati, Kokou Atchou, Délagno Irénée Kueviakoé, Mendédé Assih, Sabrina Sanvee, and Essotolom Badjabaïsi.
2023. "Quantitative Phytochemical Screening, Antioxidant and Aphrodisiac Potential Studies of Hydroethanolic Root Extract of Carissa spicatum L." Asian Journal of Biological Sciences 16, no. 2: 155-166. https://doi.org/10.3923/ajbs.2023.155.166

This work is licensed under a Creative Commons Attribution 4.0 International License.



