Wet Season Water Quality and Zooplanktons Community of Jibia Lake, Katsina State, Nigeria
| Received 31 Dec, 2022 |
Accepted 12 Mar, 2023 |
Published 30 Jun, 2023 |
Background and Objective: The quality (status of physicochemical parameters) of the freshwater ecosystem interacts with the biotic components of the ecosystem such as plankton, aquatic insects, snails and fishes. The status of freshwater qualities determines the diversity, distribution and abundance of zooplankton in the water. This study assessed the water quality of Jibia Reservoir, a manmade freshwater lake and how they interact with the zooplankton community in the lake and also provides a baseline report on the lake. Materials and Methods: Water samples were collected between July and September, 2019 at five different points of the lake for physicochemical parameters analysis and zooplankton identification using standard procedures. One-way ANOVA was used in the statistical analysis of data generated, with a 0.05 level of significance. Results: The study revealed dissolved oxygen (DO), biological oxygen demand (BOD) and total hardness (TH) and total dissolved solids (TDS) to be within the permissible limit for freshwater ecosystems. Five subclasses (Monogonata, Phyllopoda, Bdelloidea, Pterygota and Copepoda), with six families (Brachionidae, Daphnidae, Philodinidae, Curculionoidea, Canthocamptidae and Cyclopidae) of zooplanktons were recorded. Rotaria ratatoria 12 (16.90%) and Platypus quadricornis 12 (16.90%) were the most common, while Brachionus angularis 8 (11.27%) was the least common species of zooplanktons in Jibia Lake. Conclusion: The physicochemical parameters of Jibia Lake during the study period were within acceptable limits, which implies good water quality favourable to aquatic organisms. Rotaria ratatoriaandPlatypus quadricorniswere the most common zooplankton in the lake.
| Copyright © 2023 Auta et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
As an aquatic system, the freshwater ecosystem is subjected to the influence of a wide array of physical and chemical factors (physicochemical parameters), whose increase or decrease frequently affect the flora and fauna which could result in the altering of their diversity, distribution and abundance. These factors include, but not limited to pressure, density, buoyancy, temperature, light, oxygen content, dissolved oxygen, biochemical oxygen demand, carbon dioxide and pH or hydrogen ion concentration1. Waterquality affects the abundance, species composition/diversity, stability, productivity and physiological condition of indigenous populations of aquatic organisms. Some species flourish in highly eutrophic waters while others are very sensitive to organic or chemical waste2.
Zooplankton are heterotrophic planktonic animals floating in the water that constitute an important food source for many species of aquatic organisms3. In the aquatic food web of most lake ecosystems, zooplankton occupies an intermediate trophic position, inhabiting lakes with a broad range of physicochemical properties and are thus exposed to a variety of environmental threats and stressors4. When well observed, the species composition of the zooplankton can provide an indication of environmental health5. At periods of high tides, the population of some organisms may be depleted as the water quality deteriorates, which may result to the drop in the diversity of species and overall abundance of different organisms found in the water body5. The situation may turn worse when the factors impacting the communities of zooplankton remain unkown6, most especially in regions where ecological values of zooplankton are often not well appreciated7.
The interaction between the physicochemical parameters and plankton production of water bodies is of great importance in the management strategies of aquatic ecosystems8. Due to the pivotal role of zooplankton in most aquatic ecosystems, there is a constant need to explore the effect of stressors (such as the physicochemical properties of freshwater) on their diversity and abundance9.
There has been increasing concern about the rate at which inland waters are polluted through run-offs into streams and dams and the consequences on the ecosystem10. The ecological and economic importance of Jibia Lake in Jibia and environ necessitated this study, as there is a paucity of reports on its wet season water quality and zooplankton composition. Hence, this study investigated the water quality parameters and zooplankton community of Jibia Lake, as it affects their diversity, abundance and distribution in Jibia Lake.
MATERIALS AND METHODS
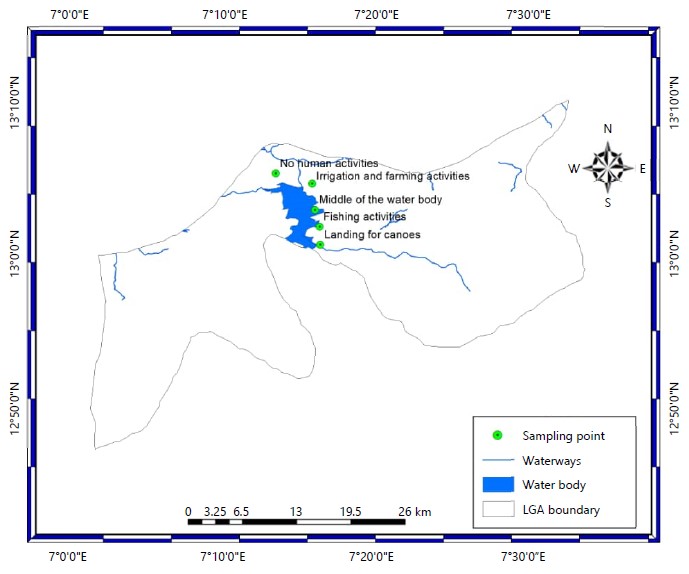
Study area: The study was conducted on Jibia Lake, located in Jibia Local Government Area of Katsina State, in North-Western Nigeria, bordering Maradi Town of Niger Republic. Jibia (Jibiya) Town is located about 43 km west of Katsina Town, in Katsina State. Its coordinates are Latitudes 13°04'18"N, 13°10'N and Longitudes 07°15'06"E, 07°.30'E (Fig. 1). The dam was designed in 1987 and completed in 1989 and was built to support irrigation activities in Jibia LGA. The landscape at the dam site is sub-desertic, except in the rainy season. The Gada River, the major tributary to the lake flows for only about four months each year. The study was done between July and September, 2019.
Sampling points: Water samples were collected from five different points in the intertidal regions of the reservoir. The choice of sampling locations of the dam was based on the ecological settings, vegetation and human activities in the area (Table 1).
Water sampling: The sampling procedures that were used during the research work involved duplicate collection of water at the dam. In this method, water was sampled at surface level by dipping 1 L plastic sampling sliding over the upper surface of the water with their mouth against the water current to permit undisturbed passage of water into the bottle, as described by Ademola et al.11.
Water sampling was done using APHA standard procedures12, where water samples for laboratory analysis were collected using pre-washed 500 mL plastic bottles. The plastic bottles were labelled on the field using appropriate codes and were temporarily stored in ice packed cooler and transported to the laboratory and stored in a refrigerator at about 40°C prior to analysis, for preservation.
|
| Table 1: | GPS coordinates of sampling points | |||
| Sampling points/station | GPS location | Human activities |
| A | LAT 13°07 05 7"N | Landing for canoes |
| LON 07°22 69 4"E | ||
| B | LAT 13°66 66 5"N | Irrigation and farming activities |
| LON 07°22 92 6"E | ||
| C | LAT 13°07 05 6"N | Fishing activities |
| LON 07°22 60 8"E | ||
| D | LAT 13°07 29 9"N | No human activities |
| LON 07°23 91 5" | ||
| E | LAT 13°06'83'5" | Middle of the water body |
| LON 07°22'73'7" |
Water samples were transported to the Biological Science laboratory at Federal University Dutsin-Ma for the analysis of physicochemical analysis of biological parameters. The samples were collected fortnightly, between July and September, 2019.
Determination of physiochemical parameters: The temperatures of water samples were measured with the use of a mercury in glass thermometer. The water pH/TDS/conductivity was taken with a pH meter (Hanna Instrument) and TDS meter (Hanna instrument) and conductivity meter (Jenway model) respectively. The nitrate and phosphate levels were analyzed by the use of a Wagtech Photometer model CP 1000. Dissolved oxygen was determined using a DO meter (Jenway model 9071) while turbidity was taken using Wagtech turbidimeter model CP1000. The total alkalinity and hardness were measured by the titration method11. Samples for biological oxygen demand were determined in the same way except that it was collected in dark reagent bottles and fixed after incubation in the dark for 5 days.
Zooplankton samples collection: Zooplankton samples were collected at each of the five sampled points. Zooplankton samples were collected using APHA method as described by Almeda et al.13 Fifty litres of water were sieved through 63 μm mesh plankton net, followed by the transfer of the filtrate into a one litre open mouth plastic container, fixed with 5% formalin. Filtrate was then taken to the laboratory for identification, using zooplankton atlas. Samples of the zooplankton were allowed to stand for a minimum of 24 hrs in the laboratory and supernatant decanted until a 50 mL concentrated residue sample was achieved.
Statistical analysis: Data obtained during the study are presented in Tables and Figures, expressed as Mean±Standard deviation. One-way Analysis of Variance (ANOVA) was used to test for difference in means of physicochemical parameters with months and points of sampling, at p<0.05 level of significance and 95% confidence interval (CI). Pearson correlation analysis was used to test for relationship among physicochemical parameters and abundance of freshwater zooplankton.
RESULTS
Water quality parameters: Results of the water quality parameters measured during the study period, in months, are presented in Table 2. The temperature was highest in September (25.01±1.63°C), with no significant difference in the mean temperature of the lake among the sampling months (p = 0.315). Mean turbidity was highest in July (466.90±58.43 NTU) and also had no significant difference of mean turbidity among the months of sampling (p = 0.525). Mean values of dissolved oxygen (DO) were highest in the September (4.46±0.55 mg L–1) and the lowest was recorded in July (1.44±0.03 mg L–1), this had a significant difference among the months of sampling (p = 0.000). Also, BOD varied significantly among the months of sampling (p = 0.000), with the highest mean values in August (2.04±0.42 mg L–1). The mean value of total hardness was highest in September (448.50±57.26 mg L–1) and lowest (255.50±88.45 mg L–1) in July, showing significant (p = 0.000) mean difference among the month samples were collected. The mean electrical conductivity (EC) was highest in July (0.55±0.01 mS cm–1) and lowest in August (0.04±0.01mS cm–1), with no significant (p = 0.235) difference among the months during which the samples were obtained. Mean value of pH was highest in August (6.51±0.43) and lowest in July (6.51±0.43), with no significant (p = 0.727) difference among the months of sampling. Mean total dissolved solids was highest in September (52.20±4.10 ppm), while the lowest value was in August (47.70±7.67 ppm) and no significant (p = 0.319) difference in mean TDS among the months.
| Table 2: | Monthly Mean±Standard deviation of the physicochemical parameters recorded in Jibia Lake | |||
| Parameter | July |
August |
September |
p-value |
| Temperature | 24.95±2.81 |
23.73±1.54 |
25.01±1.63 |
0.315 |
| Tub (NTU) | 466.90±58.43 |
445.00±42.75 |
465.00±38.071 |
0.525 |
| DO (mg L–1) | 1.44±0.03 |
4.16±0.33 |
4.46±0.55 |
0.000 |
| BOD (mg L–1) | 0.57±0.18 |
2.04±0.42 |
1.45±0.35 |
0.000 |
| TH (mg L–1) | 255.50±88.45 |
367.50±89.91 |
448.50±57.26 |
0.000 |
| EC (mS cm–1) | 0.55±0.01 |
0.04±0.01 |
0.05±0.01 |
0.235 |
| pH | 6.51±0.43 |
6.60±0.20 |
6.51±0.43 |
0.727 |
| TDS (ppm) | 49.80±7.19 |
47.70±7.67 |
52.20±4.10 |
0.319 |
| Tub: Turbidity, DO: Dissolved oxygen, BOD: Biological oxygen demand, TH, Total hardness, EC: Electrical conductivity and TDS: Total dissolved solids | ||||
| Table 3: | Gross composition of solvent extracted processed soyabean based diets | |||
| Zooplankton species | Temperature |
Tub (NTU) |
DO (mg L–1) |
BOD (mg L–1) |
TH (mg L–1) |
EC (mS cm–1) |
pH |
TDS (ppm) |
| Brachionus angularis | 0.195 |
0.161 |
0.167 |
0.227 |
0.198 |
0.48 |
0.24 |
-0.081 |
| Daphnia longispina | -0.238 |
-0.043 |
0.107 |
-0.055 |
0.064 |
0.168 |
-0.196 |
0.356 |
| Rotaria rotatoria | -0.113 |
-0.045 |
0.195 |
-0.047 |
0.262 |
0.336 |
-0.36 |
0.321 |
| Canthocamptus staphylinus | -0.088 |
0.107 |
-0.193 |
-0.249 |
-0.024 |
0.058 |
-0.074 |
0.128 |
| Platypus quadricornis | -0.007 |
-0.198 |
-0.018 |
0.112 |
0.002 |
-0.242 |
-0.207 |
0.042 |
| Eucyclops macrurus | 0.276 |
0.007 |
-0.103 |
0.074 |
0.112 |
-0.17 |
0.279 |
-0.415* |
| Thermocyclops oithnoides | 0.124 |
-0.111 |
-0.312 |
-0.17 |
-0.218 |
0.29 |
-0.15 |
-0.086 |
| *Strong correlation | ||||||||

|
|
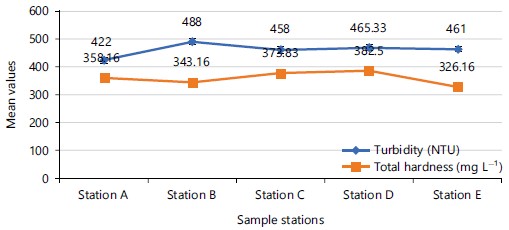
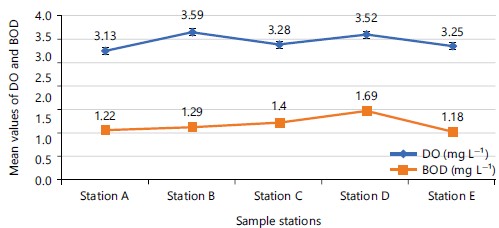
When the water quality parameters were analysed according to the points where samples were collected, the mean temperature was highest (25.80°C) at point C, while the lowest mean value (23.51°C) was at point E (Fig. 2), with no level of significance (p = 0.288) mean difference of temperature among the sampling points. Highest mean value of turbidity (488.50±22.57 NTU) was obtained at sampling point B, while its lowest value (422.00±34.64 NTU) was recorded at sampling point A and had no significant (p = 0.572) mean difference among the sampling points (Fig. 3). Mean values of total hardness (TH) was highest (382.50±56.48 mg L–1) at sampling point D and lowest (326.16±83.13 mg L–1) at sampling point E. Mean values of TH did not differ significantly (p = 0.744) among the sampling points (Fig. 2). Mean dissolved oxygen (DO) value was highest (3.59±1.70 mg L–1) at sampling point B and lowest (3.13±1.35 mg L–1) at sampling point A, with no significant (p = 0.175) difference mean value among the sampling points (Fig. 3). The mean values of biological oxygen demand (BOD) was highest (1.69±0.95 mg L–1) at sampling point D and lowest (1.18±0.52 mg L–1) at sampling point E. Mean values of BOD did not differ significantly (p = 0.427) among the sampling points (Fig. 4).
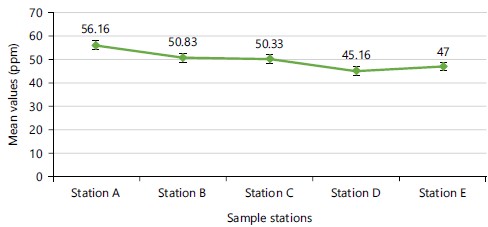
The mean values of electrical conductivity (EC) were highest (0.05±0.01 mS cm–1) at sampling points A, D and E and lowest (0.05±0.01) at sampling point C (Fig. 5), with no significant (p = 0.918) difference in the mean values among the sampling points. The mean pH values were highest (6.65±0.25) at sampling point E and lowest (6.40±0.30) at sampling point B, with no significant (p = 0.37) difference in mean values among the sampling points (Fig. 6). The mean total dissolved solids (TDS) was highest (56.16±5.74 ppm) at sampling point A and the lowest (45.16±5.15 ppm) was at sampling point D (Fig. 7). Mean values of TDS were significantly (p = 0.028) different among the sampling points.
|

|

|
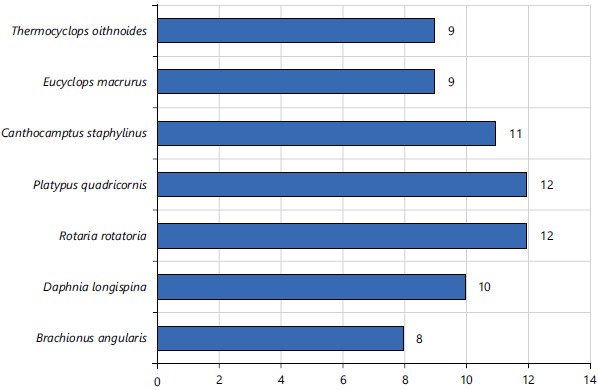
Zooplankton community in Jibia Lake: Subclasses of zooplanktons recorded in Jibia Lake during the study period had copepoda 29 (40.85%) as the most represented, while Monogonata 8 (11.27%) was the least represented subclass (Fig. 8). The families of zooplanktons most represented in Jibia Lake is Cyclopidae 18 (25.35%), while Brachionidae 8 (11.27%) was the least represented (Fig. 9). Rotaria ratatoria 12 (16.90%) and Platypus quadricornis 12 (16.90%) were the most common species of zooplanktons recorded in Jibia Lake, while Brachionus angularis 8 (11.27%) was the least common in Jibia Lake (Fig. 10).
|

|

|
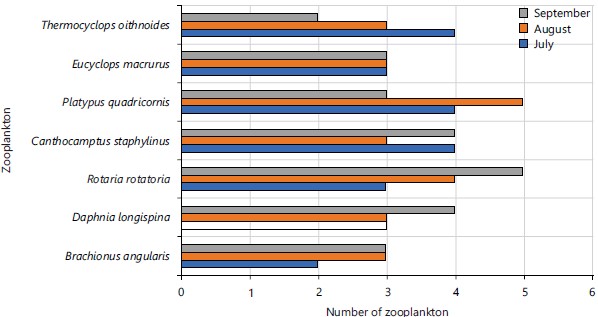
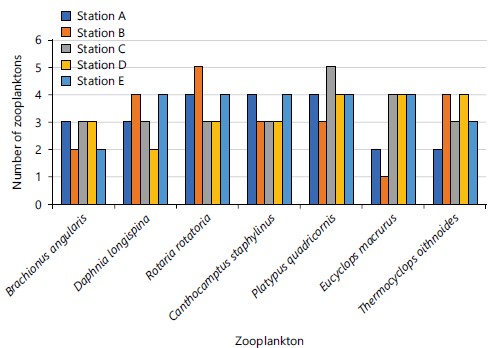
When the abundance of species was analysed with relation to the months of study, there was no significant difference in species abundance of all the zooplanktons in Jibia Lake among the months, i.e., abundance of zooplanktons did not vary significantly with the month (Fig. 11), except the E. macrurus, which significantly (0.011) varied with the months. Also, the abundance of all the zooplanktons species did not vary significantly with the sampling points at Jibia Lake (Fig. 12).
|
|
Interaction of water quality parameters with zooplanktons in Jibia Lake: Temperature shows a negative interaction with D. longispina, R. rotatoria, C. staphylinus and P. quadricornis species. Turbidity shows a negative relationship with D. longispina, R. rotatoria and P. quadricornis species. Dissolved oxygen shows negative relationship with C. staphylinus, P. quadricornis, E. macrucrus and T. oithnoides. Biological oxygen demand shows a negative relationship with D. longispina, R. rotatoria, C. staphylinus and T. oithnoides species. Total hardness shows negative relationship with C. staphylinus and T. oithnoides species. Electrical conductivity shows a negative relationship with P. quadricornis and E. macrucrus species. The pH shows a negative relationship with D. longispina, R. rotatoria, C. staphylinus, P. quadricornis and T. oithnoides. Total dissolved solids show a negative relationship with B. angularis, a strong negative relationship with E. macrucrus and a negative relationship with T. oithnoides species.
|
DISCUSSION
Findings of this study shows some dynamics in the physicochemical parameters of Jibia Lake and their interaction with the community of zooplankton. Water quality parameters (physicochemical parameters) of a freshwater ecosystem are highly dependent on the physical and geological features of the tributaries that drain into it14. The mean monthly temperature of the wet season recorded in this study is similar to the temperature reported for Zobe Dam in Katsina State, Nigeria, where the temperature was higher in July and September than in August15. It is also similar to the wet season temperature of River Jakara and Jikara Dam in Kano reported by Agbazue et al.16. This may be attributed to the peak of the rainy season in August around Katsina State, implying the rise in water volume in the lake. The mean temperature recorded at Jibia Lake differs from the average temperatures of the freshwater ecosystem reported from the southern parts of Nigeria by Oloyede et al.14, Edokpayi and Ayorinde17, Owojori et al.18 and Dunsin et al.19, who reported higher temperatures. The findings of this study, in comparison with other studies show that the water temperature of freshwater bodies in the northern part of Nigeria is lower than those of southern parts of the country. The temperature of Jibia Lake is optimal and will support zooplankton’s well-being in the water body.
The turbidity reported in this study is quite higher than the turbidity of other water bodies earlier reported by Agbazue et al.16 for River Jakara and Jakara Dam in Kano, Auta et al.15 for Zobe Dam and Oparaku et al.9 for Adada River. The high turbidity in Jibia Lake could be attributed to high turbulence which brings in clay, silt and other particles into the water. Excessive turbidity or cloudiness in drinking water is aesthetically unpleasant and may also represent a health concern16. The mean DO of Jibia Lake is similar to the DO earlier reported for River Adada by Oparaku et al.9. It was higher than the mean DO (2.707±1.327 mg L–1) of Eleyele Dam as reported by Oloyede et al.14. This is lower than the DO of River Jakara and Jikara Dam in Kano reported16. It is lower than the DO reported for Zobe Dam15. Dissolved oxygen is an indication of the degree of pollution by organic matter, the destruction of organic matter and the self-purification capacity of a water body. The standard for sustaining aquatic life is stipulated at 5 mg L–1. Thus, a concentration below this value negatively affects aquatic biological life16. Based on the lower than 5 mg L–1 DO in Jibia Lake, it implies poor support for life in the water.
The biological oxygen demand (BOD) obtained in this study is far higher than the mean BOD (0.849±0.894 mg L–1) of Eleyele Dam reported by Oloyede et al.14. Total hardness (TH) of water in Jibia Lake was far above the mean TH of River Jakara and Jikara Dam in Kano reported by Agbazue et al.16, TH of Eleyele Dam reported by Oloyede et al.14 and the TH reported for Zobe Dam in 2018 by Auta et al.15. The mean value of conductivity for Jibia Lake was far below the mean conductivity of 123.949±100.660 mS cm–1 of Eleyele Dam as was reported by Oloyede et al.14. It is also lower than the wet season conductivity of River Jakara and Jikara Dam in Kano reported by Agbazue et al.16. Electrical conductivity is an important measurement of salt content in water. High salt concentration may result in adverse effects on aquatic biota16. The low EC here implies low salt concentration in the lake.
The pH of Jibia Lake during the study was similar to the pH reported in River Adada by Oparaku et al.9, who reported a pH range of 6.57±0.1 to 7.32±0.1. It is also similar to the pH reported for Zobe Dam, which also had its highest pH in August15. Though similar to the temperature range reported for River Jakara, it is lower than the water pH of Jakara Dam in Kano16. The pH of water in Jibia Lake is lower than the average pH of 8.01±0.570 reported for Eleyele Dam by Oloyede et al.14. The low values of freshwater pH have been attributed to organic decomposition in the water body16 or partial decomposition of organic matter by bacteria and fungi that produce various organic acids that are capable of lowering the pH14. Extreme high or low pH values in freshwater have been reported to affect aquatic life and alter the toxicity of other pollutants in one form or the other. The mean total dissolved solids (TDS) of Jibia Lake were below the mean TDS reported for River Jakara and Jakara Dam in Kano reported by Agbazue et al.16 and of Eleyele Dam by Oloyede et al.14. The lower TDS in this lake may be attributed to the fact that runoffs are not deposited directly into the water body due to its embankment. It may also be attributed to the dilution effect of high volume of water16 during rainy season, as the study was carried out during wet season.
Zooplankton distribution have been reported to serve accurately as indicators of the prevailing water quality conditions of freshwater9. In this study, more copepods were recorded when compared with rotifers (Bdelloidea and monogononta put together), pterygots and phyllopods. Copepods had two families (Canthocamptidae and Cyclopidae), represented by three species (Canthocamptidae, Canthocamptus staphylinus and Cyclopidae, Eucyclops macrurus and Thermocyclops oithonoides). This finding is similar to the findings earlier reported by Oparaku et al.9, Zabbey et al.20 and Obot et al.21, who reported copepods as most abundant species in freshwaters of South East Nigeria. Their abundance could be attributed to their successful adaptation to freshwaters in the tropics and the favourable water quality parameters.
Previous reports have identified that species of copepods share some things in common with other zooplankters, such as viscous, nutritionally dilute and dangerous habitat, which have given rise to many of them evolving specialised and efficient adaptations in attempt to cope the major challenges of feeding and surviving in water. Possession of special body form which ensure efficient prey-hunting with the ability to move swiftly at high speed9. The speed ability also enhances its chances of escape from predators such as fish22.
Even though the study was limited by period of study, only physicochemical parameters analysis and morphological identification of the zooplankton using microscopy, it provides baseline data on the water quality and available zooplankton in the lake. It also sures how favourable the water condition is to the thriving of zooplankton in the water body. Hence, further research on heavy metals and organic pollutants pollution of the water and genomic variations of the zooplankton in the lake is necessary.
CONCLUSION
Analysis of water quality parameters in Jibia Lake in this study showed that its physicochemical parameters are within standard acceptable limits expected of a freshwater lake, which implies good water conditions during the wet season. Rotaria ratatoria and Platypus quadricornis were the most common zooplankton in the lake.
SIGNIFICANCE STATEMENT
In the aquatic food chain, zooplankton occupies an intermediate trophic position, that when well-studied, the species composition of the zooplankton can provide an indication of environmental health, serving as bioindicator of the freshwater ecosystem. The interaction between physicochemical parameters and zooplankton composition of water bodies is of great importance in the management strategies of aquatic ecosystems. This study is significant for providing information on the dynamics of water quality in Jibia Lake and also explaining the interaction of the water quality with the population, diversity and abundance of zooplankton. It shows that the physicochemical parameters of water in Jibia Lake is very favourable to the wellbeing of zooplankton in the lake.
REFERENCES
- Achionye-Nzeh, C.G. and A. Isimaikaiye, 2010. Fauna and flora composition and water quality of a reservoir in Ilorin, Nigeria. Int. J. Lakes Rivers, 3: 7-15
- Dorgham, M.M., 2013. Effects of Eutrophication. In: Eutrophication: Causes, Consequences and Control, Ansari, A.A. and S.S. Gill (Eds.), Springer, Dordrecht, Netherland, ISBN: 978-94-007-7813-9, pp: 29-44.
- Delince, G., 1992. The Ecology of the Fish Pond Ecosystem: With Special Reference to Africa. 1st Edn., Springer, Dordrecht, Netherlands, ISBN: 978-0-7923-1628-2, Pages: 230.
- Tartarotti, B., R. Sommaruga and N. Saul, 2022. Phenotypic and molecular responses of copepods to UV radiation stress in a clear versus a glacially turbid lake. Freshwater Biol., 67: 1456-1467.
- Chia, M.A., S.P. Bako, S.O. Alonge and A.K. Adamu, 2011. Green algal interactions with physicochemical parameters of some manmade ponds in Zaria, Northern Nigeria. Braz. J. Bot., 34: 285-295.
- Mimouni, E.A., B. Pinel-Alloul, B.E. Beisner and P. Legendre, 2018. Summer assessment of zooplankton biodiversity and environmental control in urban waterbodies on the Island of Montréal. Ecosphere, 9: e02277.
- Jeppesen, E., P. Nõges, T.A. Davidson, J. Haberman and T. Nõges et al., 2011. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia, 676: 279-297.
- Akomeah, P.A., O. Ekhator and C. Udoka, 2010. Dry Season phytoplankton composition of Ibiekuma Dam, Ekpoma, Edo State. Ethiopian J. Environ. Stud. Manage., 3: 36-40.
- Oparaku, N.F., F.A. Andong, I.A. Nnachi, E.S. Okwuonu, J.C. Ezeukwu and J.C. Ndefo, 2022. The effect of physicochemical parameters on the abundance of zooplankton of River Adada, Enugu, Nigeria. J. Freshwater Ecol., 37: 33-56.
- Bashir, I., F.A. Lone, R.A. Bhat, S.A. Mir, Z.A. Dar and S.A. Dar, 2020. Concerns and Threats of Contamination on Aquatic Ecosystems. In: Bioremediation and Biotechnology, Hakeem, K.R., R.A. Bhat and H. Qadri (Eds.), Springer, Cham, Switzerland, ISBN: 978-3-030-35691-0, pp: 1-26.
- Ademola, B.T., S.I. Mahuta, B. Abdulkarim and L.A. Argungu, 2022. Macrophytes abundance in relation to eutrophication status of peri-urban impoundments in Katsina Metropolis, Nigeria. Bayero J. Pure Appl. Sci., 13: 173-179.
- Tarekegn, M.M. and A.Z. Truye, 2018. Causes and impacts of shankila river water pollution in Addis Ababa, Ethiopia. Environ. Risk Assess. Rem., 2: 21-30.
- Almeda, R., Z. Wambaugh, Z. Wang, C. Hyatt, Z. Liu and E.J. Buskey, 2013. Interactions between zooplankton and crude oil: Toxic effects and bioaccumulation of polycyclic aromatic hydrocarbons. PLoS ONE, 8: e0067212.
- Oloyede, O.O., B. Otarigho and O. Morenikeji, 2016. Conditions of Eleyele Dam in Ibadan Nigeria inhabited by Melanoides tuberculata. Sustainability Water Qual. Ecol., 7: 22-31.
- Auta, T., E. Alkali and E.A. Michael, 2018. Population dynamics, diversity and distribution of freshwater snails in Zobe Dam, Dutsin-Ma, North-Western Nigeria. Asian J. Environ. Ecol., 8.
- Agbazue, V.E., N.R. Ekere and M.I. Samira, 2015. Physico-chemical parameters and heavy metal levels in water and fish samples from River Jakara and Jakara Dam, Kano State, Nigeria. Asian J. Chem., 27: 3794-3798.
- Edokpayi, C.A. and A.O. Ayorinde, 2005. Physical, chemical and macrobenthc invertebrate fauna characteristics of swampy water bodies within University of Lagos, Nigeria. West Afr. J. Appl. Ecol., 8.
- Owojori, O.J., S.O. Asaolu and I.E. Ofoezie, 2006. Ecology of freshwater snails in opa reservoir and research farm ponds at Obafemi Awolowo University Ile-Ife, Nigeria. J. Appl. Sci., 6: 3004-3015.
- Dunsin, B.A., C.A. Edokpayi and M.O. Lawal, 2012. Environmental conditions of a drainage channel inhabited by an invasive species Melanoids tuberculatus (Muller, 1774) in Southwestern Nigeria. Int. J. Aquat. Sci., 3: 58-70.
- Zabbey, N., F.D. Sikoki and J. Edoghotu, 2008. Plankton assemblages and environmental gradients in the middle reaches of the Imo River, Niger Delta, Nigeria. Afr. J. Aquat. Sci., 33: 241-248.
- Obot, O.I., G.S. David and I.E. Ekpo, 2020. Zooplankton assemblages of a tropical coastal creek, South-Eastern Nigeria. Ecologia, 10: 63-70.
- Kiørboe, T., 2011. What makes pelagic copepods so successful? J. Plankton Res., 33: 677-685.
How to Cite this paper?
APA-7 Style
Auta,
T., Alexander,
A., Bichi,
A.H. (2023). Wet Season Water Quality and Zooplanktons Community of Jibia Lake, Katsina State, Nigeria. Asian Journal of Biological Sciences, 16(2), 175-186. https://doi.org/10.3923/ajbs.2023.175.186
ACS Style
Auta,
T.; Alexander,
A.; Bichi,
A.H. Wet Season Water Quality and Zooplanktons Community of Jibia Lake, Katsina State, Nigeria. Asian J. Biol. Sci 2023, 16, 175-186. https://doi.org/10.3923/ajbs.2023.175.186
AMA Style
Auta
T, Alexander
A, Bichi
AH. Wet Season Water Quality and Zooplanktons Community of Jibia Lake, Katsina State, Nigeria. Asian Journal of Biological Sciences. 2023; 16(2): 175-186. https://doi.org/10.3923/ajbs.2023.175.186
Chicago/Turabian Style
Auta, Timothy, Agnes Alexander, and Armayau Hamisu Bichi.
2023. "Wet Season Water Quality and Zooplanktons Community of Jibia Lake, Katsina State, Nigeria" Asian Journal of Biological Sciences 16, no. 2: 175-186. https://doi.org/10.3923/ajbs.2023.175.186

This work is licensed under a Creative Commons Attribution 4.0 International License.



