Assessing Floristic Diversity and Ecological Characteristics of Mount Mbapit Savannah, Western Highlands of Cameroon
| Received 30 Mar, 2023 |
Accepted 15 Jun, 2023 |
Published 30 Sep, 2023 |
Background and Objective: Cameroon is one of the richest biologically diverse African countries due to its wide range of altitudes, topographic features and agroecological zones. Therefore, the aim of this study was to determine the floristic diversity and functional traits of Mount Mbapit Savannah, West Cameroon. Materials and Methods: Floristic data were collected on a total of 62 sample plots of 10×10 m between May and June, 2014, 2015 and 2022. Frequency, abundance and diversity indices were computed for the floristic diversity and life traits spectra (growth habit, life form, leaf size, diaspore type, dispersal syndromes and phytogeographical affinities). Results: In total, 144 plant species (91 herbaceous and 53 woody) belonging to 110 genera and 50 families were identified. The most abundant families were Poaceae (28 species) and Asteraceae (20 species). The species diversity indices were Shannon-Weaver (4.92 and 3.61) and evenness index (0.73 and 0.62) for herbaceous and woody species, respectively. The most represented life forms were phanerophytes (43.75%). Anemochory (45.83%) was the dominant dispersal syndrome. Phytogeographical distribution analysis showed the predominance of afro-tropical species. Conclusion: Appropriate conservation measures such as assisted natural regeneration and increased protection should be taken for the threatened species.
| Copyright © 2023 Junior Baudoin et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The flora of vascular plants flora of Cameroon is estimated at 7,884 taxa and ranks fourth among the richest African countries in floristic biodiversity with 585 plant species known only in Cameroon1. The Cameroon Highlands are formed by an interrupted volcanic line and contain Africa’s highest mountains west of the Albertine Rift, namely Mount Cameroon, Mount Oku, Mount Bamboutos and Mount Mbapit, which are part of the 25 ‘biodiversity hotspots’ defined as priorities in the planet scale for the conservation of biodiversity2 peaking at 1988 m, this mountain has an attractive Crater Lake. The ecosystem of the Mount Mbapit has been degraded mainly by human activities through uncontrolled farming, overgrazing, cutting down trees for firewood, bushfires, an increasing need for agricultural land which makes people clear the forest, leaving the bare soils and natural factors such as climate change, soil erosion and the presence of invading species. This tropical mountain once covered by mountain forests has been suddenly changed into savannah.
Floristic composition and ecological diversity are among the most significant ecological attributes of a particular plant communities, which show variations in response to biotic and abiotic factors3,4. Such information is useful not only in understanding plant diversity and the ecological functions of plant communities but also in providing insight into the environmental requirements of the plant species to adapt in specific habitats5. Studying functional plant traits of a given area is an important tool that helps in understanding the relationship between plant community structure, environmental factors and distribution, ultimately revealing the ecological functions of individual species in a community6. Savannahs, compared to the forest, are easier to convert to crops and their degradation is not considered as a serious environmental attack compared to the clearing of forests7,8. These ecosystems have often been noticed as the epicentres of different destructive bushfires that, along the history of Mount Mbapit have decimated large tracts of other habitats such as the closed evergreen forest and bamboo areas. Floristic knowledge, from several authors in the Western Highlands Region of Cameroon6-7,9-14, showed high floristic diversity. These studies do not cover the entire region. Thus, the current knowledge of these tropical mountain savannahs lacks quantitative data, which are particularly crucial for estimating the floristic diversity and the functional traits of plant communities and for ensuring good management of the plant resources.
This study is a good contribution to describing the floristic diversity and the ecological characteristics of the plant communities in order to provide scientific data for the conservation and sustainable management of Mount Mbapit Savannahs biodiversity. Such information is useful not only for understanding the impact of altered environmental conditions on plant community structure, but also for providing insight into the environmental requirements of species necessary for successful ecological restoration and biodiversity protection. The aim of this study was to assess the phytodiversity and functional traits of the savannahs of Mount Mbapit, West Cameroon.
MATERIALS AND METHODS
Study area: This study was carried out in Mount Mbapit which is located in the Noun Division of the West region of Cameroon between the localities of Foumbot and Foumban (5°40'-5°52'N and 10°30'-10°45'E). The study was carried out from May to June, 2014, 2015 and 2022. This mountain with its maximum height of 1988 m. The climate is defined as tropical equatorial-guinean type characterized by a rainy season which extends from March to November and a dry season from December to February. The average annual precipitation was 2500 mm while the mean annual temperature was 21°C. A dry season occurs between November and March and a rainy season takes place between March and November. The predominant soils are vitric andosols. The predominant ecosystem is open grassland with trees and shrubs at some points. The vegetation is a savannah dominated by Pennisetum purpureum and Imperata cylindrica in the herbaceous layer with ligneous cover highly modified by human activities such as agriculture, bushfire, grazing and wood collection7,9.
Sampling: Floristic inventory was carried out during the months of May and June, 2014, 2015 and 2022. Plant species were inventoried in 10×10 m (100 m²) plots and equidistant of at least 200 m. Trees and shrubs were named and encountered within plots of 100 m² and herbs within 5 subplots of 1 m² placed within each 100 m² plot (4 in each corner 100 m² plot and one in the centre). In total, 62 plots of 100 m² and 310 of 1 m² were sampled in the present study. The sampling of herbaceous vegetation was done according to the Braun-Blanquet method using mixed sampling. Some plants species were identified directly in the field using monographs, for the other species, specimens were collected and compared which those available at the National Herbarium of Cameroon. Farming lands were not examined. The plant nomenclature system of family adopted was Angiosperms Phylogeny Group 4 (APG IV) classification.
The growth habit of the plant species was determined in the field by observation of the plants. The types of life forms allowed to determine adaption strategies as well as the physiognomy of the vegetation were defined and classified according to Raunkiaer15. Leaves sizes were also determined and classified according to Ohsawa16. The types of diaspores which show the ability of plants to disperse, regenerate and establish in the ecosystem were defined and classified according to the classification of Dansereau and Lems17. The dispersal type which shows the ability to migrate, colonize new environments and dispersal distance, were identified using the morphological attributes developed by the species to disperse the seeds17. The phytogeographic type which shows flora stability and age, response to natural disturbances and to human presence (species exchanges between continents) and response to isolation and forest degradation (endemic species), adopted correspond to the major chronological subdivisions retained for Africa according to Gonmadje18.
Data processing and statistical analysis: Floristic composition was assessed on the basis of species richness, genus and family, abundance and frequency of species. The floristic diversity was calculated using PAST 3.0 software and according to several indices that take into account both the number of species and their dominance cover-abundance: Shannon-Weaver diversity index:
where, ni is the number of individuals of species, i and N is the total number of individuals of all species. Pielou evenness index:
where, S is the total number of species. Simpson’s diversity index:
The Margalef index:
where, S is the total number of species and N is the total number of individuals for all species.
The proportions of each ecological spectrum were also calculated. All data were subject to descriptive statistical analysis using Microsoft Excel 2017 software.
RESULTS
Plant species composition and diversity: A total of 144 plant species (91 herbaceous and 53 woody) belonging to 110 genera and 50 families (APG IV) were reported in the current study area (Table 1). Four species remained determined only to the genus level while five species, represented by only one individual each, remained undetermined. The most frequent herbaceous species were: Imperata cylindrica (present in 68.6% of all the plots), followed by Asplenuim abyssinicum (54.2%) and Vernonia guinensis (48.6%). In terms of cover-abundance, the most dominant herbaceous species were Imperata cylindrica (20.2% of the total area), Hypparhenia barcteata (14.6%), Pennisetum polystachion (8.2%), Chromolena odorata (5.4%), Digitaria debilis (3.4) and Asplenuim abyssinicum (2.9%). The richest genus was Ficus with 6 species followed by Digitaria, Pennisetum, Psorospermum with 4 species each and Dissotis, Helichrysum, Panicum, Vernonia with 3 species each.
| Table 1: | Floristic composition and functional traits of the savannah vegetation of the mount Mbapit | |||
| Families | Species name | IUCN status |
Growth form |
Raunkiaer life-form |
Types of diaspores |
Dispersal syndromes |
Leaf size |
Phytochoria |
| Amaranthaceae | Cyathula cylindrica (Moq.) | LC |
Herb |
Ch |
Ballo |
Auto |
Mi |
Afro-Trop |
| Cyathula uncinulata (Schard.) Schinz | LC |
Herb |
Ch |
Desmo |
Zoo |
Mi |
AM |
|
| Mangifera indica L. | LC |
Tree |
MaPh |
Baro |
Auto |
Mg |
Pan |
|
| Annonaceae | Annona senegalensis Pers. | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Plur-Afr |
| Apocynaceae | Mondia whitei (Hook. f.) Skeel | NE |
Liana |
PhL |
Sarco |
Zoo |
Me |
Afro-Trop |
| Araliaceae | Cussonia arborea Hochst. ex. A. Rich | LC |
Tree |
MaPh |
Sarco |
Zoo |
Mg |
Afro-Trop |
| Polyscias fulva (Hiern) Harms | LC |
Tree |
MaPh |
Sarco |
Zoo |
Me |
Mo (Afrtrop) |
|
| Schefflera abyssinica (Hochst. ex. A. Rich) Harms | LC |
Tree |
MaPh |
Sarco |
Zoo |
Ma |
Mo (Afrtrop) |
|
| Arecaceae | Elaeis guineensis Jacq. | LC |
Tree |
MsPh |
Sarco |
Zoo |
Mg |
Pan |
| Phoenix reclinata Jacq. | LC |
Tree |
MsPh |
Sarco |
Zoo |
Mg |
Pan |
|
| Asclepiadaceae | Brachystelma omissum Bullock | VU |
Herb |
Ge |
Sclero |
Ane |
Me |
Cos |
| Margaretta rosea Oliv | LC |
Herb |
McPh |
Sarco |
Zoo |
Mi |
Cos |
|
| Asparagaceae | Asparagus racemosus Willd. | DD |
Shrub |
McPh |
Ind |
Ind |
Me |
Pan |
| Aspleniaceae | Asplenium abyssinicum Fée | LC |
Herb |
Ge |
Sporo |
Ane |
Me |
Pan |
| Asplenium mannii Hook. | LC |
Herb |
Ge |
Sporo |
Ane |
Me |
Cos |
|
| Asteraceae | Acanthospermum brasilum Schrank | LC |
Herb |
Ch |
Ballo |
Auto |
No |
AA |
| Ageratum conyzoides Linn. | LC |
Herb |
Th |
Pogo |
Ane |
No |
Pan |
|
| Aspilia africana (Pers.) C.D. Adams | LC |
Herb |
Nph |
Pogo |
Ane |
Mi |
Plur-Afr |
|
| Bidens pilosa (L.) | LC |
Herb |
Th |
Pogo |
Ane |
Me |
Pan |
|
| Chromolaena odorata (L.) R. M. King and H. Rob. | LC |
Shrub |
Nph |
Pogo |
Ane |
Me |
Pan |
|
| Conyza sumatrensis (Retz.) E. Walker | LC |
Herb |
Ch |
Pogo |
Ane |
Mi |
Pan |
|
| Coreopsis carupokum Hutch | LC |
Herb |
Nph |
Pogo |
Ane |
Me |
Afro-Trop |
|
| Crassocephalum bauchiense (Hutch.) Milne-Redh. | NT |
Herb |
Ch |
Pogo |
Ane |
Me |
Mo (DC) |
|
| Crassocephalum gracile (Hook. f.) Milne-Redh. | LC |
Herb |
Ch |
Pogo |
Ane |
Me |
Mo (DC) |
|
| Echinops gracilis (O. Hoffen) | LC |
Herb |
Ch |
Pogo |
Ane |
Na |
Afro-Trop |
|
| Emilia coccinea (Sims) G. Don | LC |
Herb |
Ch |
Pogo |
Ane |
No |
Pan |
|
| Helichrysum antunesi Volkens and O. Hoffm. | LC |
Herb |
Th |
Pogo |
Ane |
No |
Mo (DC) |
|
| Helichrysum mechowianum Klatt | LC |
Herb |
Th |
Pogo |
Ane |
No |
Afro-Trop |
|
| Helichrysum sp. | LC |
Herb |
Th |
Pogo |
Ane |
No |
Ind |
|
| Laggera pterodonta (DC.) Sch. Bip. ex. Oliv. | LC |
Herb |
Ch |
Pogo |
Ane |
No |
Pan |
|
| Microglossa angolensis | LC |
Shrub |
MsPh |
Pogo |
Ane |
Me |
Pal |
|
| Tithonia diversifolia A. Gray (Nat.) | LC |
Shrub |
Nph |
Sclero |
Ane |
Me |
Pan |
|
| Vernonia acrocephala (Klatt) | NT |
Herb |
Nph |
Pogo |
Ane |
Mi |
SZ |
|
| Vernonia amygdalina (Delile) | LC |
Shrub |
McPh |
Pogo |
Ane |
Me |
Plur-Afr |
|
| Balsaminaceae | Impatiens burtonii Hook. f. | LC |
Herb |
Ch |
Sarco |
Zoo |
Me |
SZ |
| Cannabaceae | Celtis africana Burm. f. | LC |
Tree |
MsPh |
Sarco |
Zoo |
Me |
Afro-Trop |
| Capparaceae | Maerua pseudopetalosa (Gil and Gil-Ben) de Wolf | LC |
Shrub |
McPh |
Ballo |
Auto |
Mi |
Afro-Trop |
| Cleome iberidella Welw. ex. Oliv. | LC |
Herb |
Ch |
Ballo |
Auto |
Me |
Mo (Afrtrop) |
|
| Combretaceae | Terminalia glaucescens Planch. ex. Benth. | LC |
Tree |
MaPh |
Ptero |
Ane |
Me |
SZ |
| Commelinaceae | Commelina benghalensis L. | LC |
Herb |
Ch |
Ballo |
Auto |
Mi |
Pal |
| Cyanolis mannii C.B. Clarcke | LC |
Herb |
Ch |
Ballo |
Auto |
Mi |
Afro-Trop |
|
| Convolvulaceae | Ipomoea involucrata P. Beauv. | LC |
Liana |
He |
Baro |
Auto |
No |
Pans |
| Costaceae | Costus spectabilis (Fenzl) K. Schumann | LC |
Herb |
Ge |
Sarco |
Zoo |
Me |
SZ |
| Crassulaceae | Crassula mannii (Hook. f.) | LC |
Herb |
Ch |
Sclero |
Ane |
Mi |
Afro-Trop |
| Cyperaceae | Cyperus difformis L. | LC |
Herb |
Ge |
Ptéro |
Ane |
No |
Pan |
| Cyperus distans (Linn. F) | LC |
Herb |
Ge |
Ptéro |
Ane |
No |
Pan |
|
| Dennstaediaceae | Microlepia speluncae (L.) T. Moore | LC |
Herb |
He |
Sporo |
Ane |
No |
Pan |
| Pteridium aquilinum (L.) Kuhn | LC |
Herb |
Ge |
Scléro |
Ane |
Ma |
Cos |
|
| Dryopteridaceae | Ctenitis sp. | Ind |
Herb |
Ge |
Sclero |
Ane |
Me |
Ind |
| Euphorbiaceae | Alchornea cordifolia (Schumach. and Thonn.) Mll. Arg. | LC |
Herb |
PhL |
Pogo |
Ane |
Me |
GC |
| Euphorbia hirta (L.) | LC |
Herb |
Th |
Sarco |
Zoo |
Mi |
Pan |
|
| Manihot esculenta Crantz | LC |
Shrub |
MsPh |
Baro |
Auto |
Me |
Pan |
|
| Fabaceae | Calopogonium mucunoides Desv | LC |
Herb |
Ch |
Sclero |
Ane |
Me |
Pan |
| Crotalaria astragalina Hochst. | LC |
Herb |
Nph |
Ballo |
Auto |
Mi |
Afro-Trop |
|
| Cyclocarpa stellaris (Afzel and buk) | LC |
Herb |
Nph |
Ballo |
Auto |
Mi |
Pal |
|
| Daniellia oliveri (Rolfe) Hutch. and Dalziel | LC |
Tree |
MaPh |
Ballo |
Auto |
Me |
GC |
|
| Desmodium adscendens (Sw.) D.C. | LC |
Herb |
Th |
Desmo |
Zoo |
Mi |
Pan |
|
| Entada africana (Guill and Perr.) | LC |
Tree |
MaPh |
Ballo |
Auto |
Ma |
SZ |
|
| Eriosema bauchiense (Huth. R.) Dalg | NT |
Herb |
Ge |
Ballo |
Auto |
No |
Afro-Trop |
|
| Eriosema glomeratum Hook. f. | LC |
Herb |
Ge |
Ballo |
Auto |
No |
Plur-Afr |
|
| Erythrina senegalensis A.D.C. | LC |
Shrub |
McPh |
Ballo |
Auto |
Ma |
SG |
|
| Indigofera mimozoides (Bak.) | LC |
Herb |
Nph |
Ballo |
Auto |
Mi |
Afro-Trop |
|
| Mimosa pigra (Linn.) | LC |
Herb |
Ch |
Ballo |
Auto |
Na |
Pan |
|
| Mimosa pudica L. | LC |
Herb |
Nph |
Pogo |
Ané |
Mi |
Cos |
|
| Mucuna stans (Welw. and Bak) | LC |
Shrub |
Nph |
Ballo |
Auto |
Mi |
SZ |
|
| Piliostigma thonningii (Schumach.) Milne-Redh. | LC |
Shrub |
MsPh |
Ballo |
Auto |
Me |
SZ |
|
| Stylosanthes hamata (L.) Taub. | LC |
Herb |
Ch |
Ballo |
Auto |
No |
Cos |
|
| Hypericaceae | Harungana madagascariensis Lam. ex. Poir. | LC |
Shrub |
MsPh |
Sarco |
Zoo |
Me |
Afro-Trop |
| Hypericum lanceolatum Lam | LC |
Shrub |
McPh |
Ballo |
Auto |
No |
Mo (Afrtrop) |
|
| Hypericum quartinianum A. Rich. | LC |
Shrub |
McPh |
Ballo |
Auto |
No |
Mo (Afrtrop) |
|
| Psorospermum cf senegalense (Stems and Le) | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Psorospermum corymbiferum Hochr. | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Psorospermum febrifugum Spach | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
SZ |
|
| Psorospermum ferruginea (Le) | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Lamiaceae | Leucas oligocephala (Hook. f.) | LC |
Herb |
Ch |
Sclero |
Ane |
Mi |
Afro-Trop |
| Vitex. grandifolia Gürke | LC |
Tree |
MsPh |
Sarco |
Zoo |
No |
Afro-Trop |
|
| Lauraceae | Persea americana Mill. | LC |
Tree |
MaPh |
Baro |
Auto |
Me |
Pan |
| Malvaceae | Pavonia schimperiana Hoscht. | LC |
Shrub |
Nph |
Desmo |
Zoo |
Mi |
Afro-Trop |
| Sida acuta Burm. F. | LC |
Herb |
Nph |
Acan |
Zoo |
Mi |
Pan |
|
| Sida corymbosa (R.E.) Fries | LC |
Herb |
Th |
Desmo |
Zoo |
Mi |
Pan |
|
| Urena lobata L. | LC |
Herb |
Ch |
Desmo |
Zoo |
No |
Pan |
|
| Melastomataceae | Dissotis phaeotricha (Hochst.)Hook. f. | LC |
Herb |
Ch |
Sarco |
Zoo |
Me |
Afro-Trop |
| Dissotis princeps (Bompl.)triana | LC |
Herb |
Ch |
Sarco |
Zoo |
No |
Afro-Trop |
|
| Dissotis thollonii Coginiaux et Buttner | LC |
Herb |
Ch |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Meliaceae | Carapa grandiflora Sprague | NE |
Tree |
MaPh |
Sarco |
Zoo |
Me |
Mo (Afrtrop) |
| Moraceae | Ficus alata (L). | LC |
Tree |
MaPh |
Sarco |
Zoo |
Me |
Pal |
| Ficus ex. asperate | LC |
Tree |
MsPh |
Sarco |
Zoo |
Me |
Pal |
|
| Ficus mucuso Ficalho | LC |
Tree |
MaPh |
Sarco |
Zoo |
Me |
GC |
|
| Ficus sycomorus L. | LC |
Tree |
MsPh |
Sarco |
Zoo |
Me |
Pal |
|
| Ficus thonningii Blume | LC |
Tree |
MsPh |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Ficus vallis-choudae Delile | DD |
Shrub |
MaPh |
Sarco |
Zoo |
Me |
SG |
|
| Musaceae | Ensete livingstonianum (J. Kirk) Cheesman | LC |
Shrub |
Ch |
Ballo |
Auto |
Mg |
SZ |
| Myrtaceae | Eucalyptus saligna Hort. Berol ex. Maiden | LC |
Tree |
MaPh |
Ballo |
Auto |
Me |
Pal |
| Psidium guajava (L.) | LC |
Tree |
MaPh |
Sarco |
Zoo |
Me |
Pan |
|
| Ochnaceae | Lophira lanceolata Tiegh. ex. Keay | LC |
Tree |
MaPh |
Ptero |
Ane |
Me |
GC |
| Onagraceae | Ludwigia abyssinica (A. Rich.) | LC |
Herb |
Th |
Sclero |
Ane |
Mi |
Plur-Afr |
| Orchidaceae | Disa nigerica (Rolf.) | LC |
Herb |
Ch |
Sclero |
Ane |
No |
Afro-Trop |
| Oxalidaceae | Oxalis corniculata L. | LC |
Herb |
Th |
Ballo |
Auto |
Na |
Cos |
| Phyllanthaceae | Bridelia ferruginea Benth. | LC |
Shrub |
MsPh |
Sarco |
Zoo |
Me |
Afro-Trop |
| Bridelia scleroneura Müll. Arg. | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Pal |
|
| Phyllanthus muellerianus (Kuntze) ex. ell | LC |
Shrub |
MsPh |
Ballo |
Auto |
Na |
Afro-Trop |
|
| Pittosporaceae | Pittosporum mannii Hook. f. | LC |
Shrub |
MsPh |
Sarco |
Zoo |
Me |
MO (D.C.) |
| Poaceae | Andropogon lima (Hackel) Stapf | LC |
Herb |
He |
Sclero |
Ane |
Me |
Pal |
| Andropogon mannii Hooker f. | LC |
Herb |
He |
Sclero |
Ane |
Me |
Pal |
|
| Digitaria adamaouensis Van der Zon | EN |
Herb |
Th |
Sclero |
Ane |
Mi |
Afro-Trop |
|
| Digitaria debilis (Desfontaines) Willdenow | LC |
Herb |
Ge |
Sclero |
Ane |
Mi |
GC |
|
| Digitaria diagonalis (Nees) Stapf | LC |
Herb |
Th |
Sclero |
Ane |
Me |
Afro-Trop |
|
| Digitaria uniglurnis Stapf. | LC |
Herb |
Ge |
Sclero |
Ane |
Mi |
Afro-Trop |
|
| Diheteropogon grandiflorus Stapf | LC |
Herb |
Ge |
Sclero |
Ane |
Me |
Afro-Trop |
|
| Eleusine indica (Linne) Gaertner | LC |
Herb |
Th |
Sclero |
Ane |
No |
Pan |
|
| Festuca abyssinica A. Richard | LC |
Herb |
Th |
Sclero |
Ane |
Me |
Mo (Afrtrop) |
|
| Helictotrichon elongatum (A. Rich.) C.E. Hubbard | LC |
Herb |
Th |
Sclero |
Ane |
Na |
Mo (DC) |
|
| Heteropogon contortus (L.) Roem. and Schult | LC |
Herb |
Th |
Pogo |
Ane |
Na |
Pal |
|
| Hyparrhenia bracteata (Humb. and Bonpl.) Stapf | LC |
Herb |
He |
Sclero |
Ane |
Na |
Afro-Trop |
|
| Hyparrhenia rufa Stapf | LC |
Herb |
He |
Sclero |
Ane |
Na |
Pan |
|
| Imperata cylindrica (L.) Raeuschel. | LC |
Herb |
Ge |
Scléro |
Ané |
No |
Pan |
|
| Loudetia camerunensis (Stapf) C.E. Hubbard | LC |
Herb |
He |
Sclero |
Ane |
No |
Afro-Trop |
|
| Melinis repens (Willdenow) Zizka | LC |
Herb |
Th |
Sclero |
Ane |
Mi |
Pan |
|
| Panicum brevifolium L. | LC |
Herb |
Ch |
Sclero |
Ane |
Mi |
Pal |
|
| Panicum hochstetteri Steudel | LC |
Herb |
He |
Sclero |
Ane |
Mi |
MO (DC) |
|
| Panicum laxum (Swartz) | LC |
Herb |
He |
Sclero |
Ane |
Mi |
Pan |
|
| Pennisetum clandestinum Chiovenda | LC |
Herb |
Th |
Sclero |
Ane |
Me |
Pan |
|
| Pennisetum polystachion (Linne) Schultes | LC |
Herb |
Th |
Sclero |
Ane |
Me |
Pan |
|
| Pennisetum purpureum Schumacher | LC |
Herb |
Th |
Sclero |
Ane |
Mi |
Afro-Trop |
|
| Pennisetum unisetum (Nees) Bentham | LC |
Herb |
Th |
Sclero |
Ane |
No |
SZ |
|
| Raphia mambillensis Otedoh | LC |
Shrub |
McPh |
Sarco |
Zoo |
Mg |
AM |
|
| Setaria barbata (Lam.) Kunth | LC |
Herb |
He |
Sclero |
Ane |
Ma |
Cos |
|
| Sporobolus pyramidalis P. Beauv. | LC |
Herb |
Th |
Sclero |
Ane |
Mi |
AM |
|
| Sporobolus subulatus Hackel | LC |
Herb |
Th |
Sclero |
Ane |
Mi |
AM |
|
| Stenotaphrum secundatum (Walter) Kuntze | LC |
Herb |
Ch |
Ballo |
Auto |
Mi |
Cos |
|
| Primulaceae | Maesa lanceolata (Forssk.) | LC |
Shrub |
McPh |
Sarco |
Zoo |
Me |
Afro-Trop |
| Proteaceae | Protea madiensis Oliv. | LC |
Shrub |
Mcph |
Pogo |
Ane |
Me |
SZ |
| Ranunculaceae | Clematis altissima Hutch. | LC |
Liana |
PhL |
Pogo |
Ane |
Mi |
Pal |
| Rosaceae | Prunus africana (Hook. f.) Kalkman | NT |
Tree |
MsPh |
Sarco |
Zoo |
Me |
Mo (Afrtrop) |
| Rubiaceae | Coffea sp. | Und |
Shrub |
MsPh |
Baro |
Auto |
Me |
Ind |
| Fadogia sp. | Und |
Herb |
Ch |
Sarco |
Zoo |
No |
Ind |
|
| Sarcocephalus latifolius (Sm.) E.A. Bruce | LC |
Shrub |
MsPh |
Sarco |
Zoo |
Me |
Afro-Trop |
|
| Spermacoce pusilla (Wall.) | LC |
Herb |
Ch |
Ballo |
Auto |
Mi |
SZ |
|
| Rutaceae | Fagara leprieurii (Guil. and Perr) Engl. | LC |
Shrub |
Th |
Sarco |
Zoo |
No |
Pal |
| Sapotaceae | Vitellaria paradoxa C.F. Gaertn. | VU |
Tree |
MaPh |
Sarco |
Zoo |
No |
SZ |
| Verbenaceae | Lippia adoensis (Hoscht) | LC |
Herb |
Nph |
Ptero |
Ane |
Mi |
AA |
| Woodsiaceae | Diplazium sammatii (Kuhn) C. Chr | LC |
Herb |
Ge |
Sporo |
Ane |
Ma |
Afro-Trop |
| Zingiberaceae | Aframomum daniellii (Hook. f.) K. Schum. | LC |
Herb |
Ge |
Sarco |
Zoo |
Mg |
GC |
| IUCN status: LC: Least concern, EN: Endangered, VU: Vulnerable, NT: Near threatened. DD: Data deficient, Life forms Th: Therophytes, He: Hemicryptophytes, MaPh: Macrophanerophytes, NnPh: Nanophanerophytes, Ch: Chamaephytes, Ge: Geophytes, MsPh: Mesophanerophytes, McPh: Microphanerophytes, Diaspores types: Acan: Acanthochores, Ballo: Ballochores, Pogo: Pogonochores, Baro: Barochores, Desmo: Desmochores, Ptéro: Pterochores, Sarco: Sarcochores, Scléro: Sclerochores, Sporo: Sporochores, Und: Undetermined, Dispersal syndrome: Ane: Anemochory, Zoo: Zoochory, Auto: Autochory, Leaf size: Mg: Megaphyll, Ma: Macrophyll, Mé: Mesophyll, No: Notophyls, Mi: Microphyll, Na: Nanophyll, Phytogeographical affinities: AA: Afro-American, Afro-Afro-Trop: Tropical, AM: Afro-Malagasy, Cos: Cosmopolitan, GC: Guineo-Congolian, Pal: Paleotropical, Pan: Pantropical, Plur-Afr: Pluriregional African, SG: Sudano-guinean, SZ: Sudano-Zambezian, MO (DC): Only in Cameroonian mountain, Mo (Afrtrop): Afro-Tropical mountains and Und: Undetermined | ||||||||
In total, 2033 individuals belonging to 53 woody species were recorded on the study site (Table 1). The most frequent species were Terminalia glaucescens (50%), Annona senegalensis (41.66%), Cussonia arborea 33.33%) and Entada africana (33.33%). The most abundance woody species were Tithonia diversifolia (14.46%), Piliostigma thonningii (9.24%), Annona senegalensis (6.64%) and Protea madiensis (6.24%).
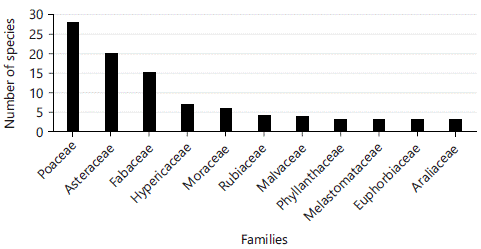
The most represented families were Poaceae (28 species, 19.58%), Asteraceae (20 species, 13.98%), Fabaceae (15 species, 10.48%), Hypericaceae (7 species, 4.89%) and Moraceae (6 species, 4.19%) (Fig. 1). The eleven richest families accounted for 96 species (67.61%). However, in terms of cover-abundance, the most abundant families are Poaceae (covering 49.8%), Asteraceae (covering 15.6%), Aspleniaceae (12.5%) and Fabaceae (4.8%).
The Shannon-Weaver diversity index was 4.92 and 3.61, the Pielou evenness index was 0.73 and 0.62, the Simpson index was 0.07 and 0.12, respectively for herbaceous and woody species. The Margalef species richness was 11.12 species per hectare.
Species conservation status: The conservation status of 144 plants was assessed and seven were found important for conservation in the study area. This assessment showed that Digitaria adamaouensis is Endangered, Brachystelma omissum, Crassocephalum bauchiense, Vitellaria paradoxa are Vulnerable and Eriosema bauchiense, Prunus africana, Vernonia acrocephala are near threatened.
FUNCTIONAL TRAITS
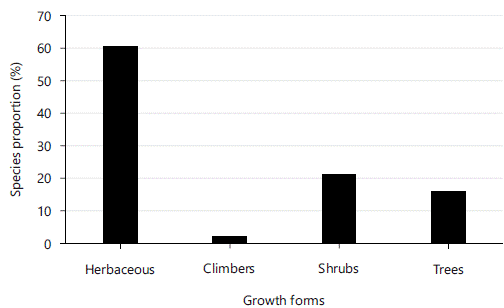
Plant growth forms: In the current study, the highest number of plant species (87) were Herbaceous plants (60.83% of all species), followed by 31 shrubs species (21.52%), 21 trees (15.97%) and three climbers (2.03%) (Fig. 2).
|
|
|
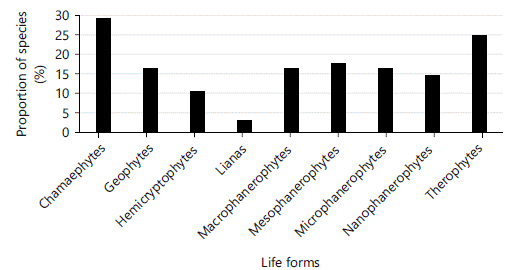
Life forms: About 43.75% of the species (63 species) were phanerophyte which constituted the dominant life form, followed by 28 species of chamaephytes (19.44%), 24 species of therophytes (16.67%) and 16 species geophytes with (11.11%) (Fig. 3). The least represented life forms were hemicryptophytes with 10 species (6.94%) and lianas with 3 species (2.08%). The detailed phanerophyte life form showed a high proportion of mesophanerophytes with 16 species (11.80%), macrophanerophytes and microphanerophytes with 17 species each (11.11%) and nanophanerophytes with 14 species (09.72%).
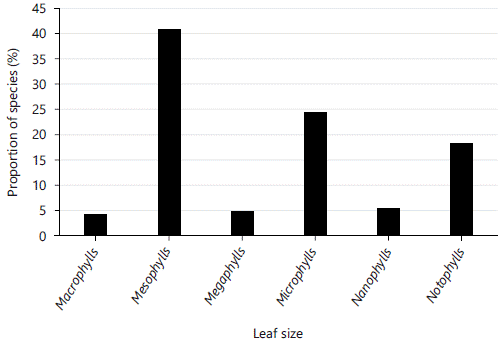
Leaf size spectrum: The most common leaf size was mesophylls with 60 species (41.66%), followed by microphylls 36 species (25%) and notophylls 27 species (18.75%) (Fig. 4). Species with large leaves sizes (megaphylls and macrophylls) and small leaf size (nanophylls) were less in abundant. Aphyllous species were absent.
Type of diaspores and their dispersal syndromes: The ability of species to colonize new sites, regenerate and persist locally showed that sarcochores (27.78%) were the most dominant diaspores type. This was followed by sclerochores (23.61%), ballochores (18.06%) and pogonochores (15.97%) (Table 2). The majority of taxa in the study plots were dispersed by wind (anemochorous species, 45.83%), followed by zoochory (31.94%) and the least was autochory (22.22%).
Phytogeographic affinities: The survey of the geographical distribution of plants species indicated that the total flora was composed mainly of Afro-Tropical species (25.69%) followed by Pantropical species (22.92%), Sudano-Zambezian (9.72%) and Paleotropical (9.72%) (Table 3). Afro-American and Sudano-Guinean species were the least represented with 1.39% each of the total species recorded.
|
| Table 2: | Proportion of species with different types of diaspores and dispersal syndromes | |||
| Diaspores types | Dispersal syndrome |
Species number |
Proportion (%) |
Anemochory |
66 |
45.83 |
|
| Sclerochores | 34 |
23.61 |
|
| Pterochores | 5 |
3.47 |
|
| Pogonochores | 23 |
15.97 |
|
| Sporochores | 4 |
2.77 |
|
Zoochory |
46 |
31.94 |
|
| Acanthochores | 1 |
0.7 |
|
| Sarcochores | 40 |
27.78 |
|
| Desmochores | 5 |
3.47 |
|
Autochory |
32 |
22.22 |
|
| Ballochores | 26 |
18.06 |
|
| Barochores | 5 |
3.47 |
|
| Undetermined | 1 |
0.7 |
|
| Table 3: | Geographic distribution of plants showing the proportion of species in each chorotype | |||
| Phytogeographic affinities | Proportion (%) |
| Afro-American | 1.39 |
| Afro-Tropical | 25.69 |
| Afro-Malagasy | 2.78 |
| Cosmopolitan | 6.25 |
| Guineo-Congolian | 4.16 |
| Paleotropical | 9.72 |
| Pantropical | 22.92 |
| Pluriregional African | 3.47 |
| Sudano-Guinean | 1.39 |
| Sudano-Zambezian | 9.72 |
| Only in Cameroonian Mountains | 4.19 |
| Undetermined | 2.78 |
| Afro-tropical mountains | 5.55 |
DISCUSSION
Mount Mbapit is rich and diverse in plant species with 144 plant species belonging to 110 genera and 50 families recorded. Several factors including geomorphological (slope zones), edaphic (soil types) and climatic conditions can explain the high species richness of this mountain. The number of plant species recorded in this survey was lower than the 209 species belonging to 139 genera and 63 families reported by Wouokoue et al.9 in the Bambouto mountains but was higher than the 121 plant species belonging to 91 genera and 34 families reported by Mbogue et al.11 in the Western Highlands of Cameroon. The factors determining the spatial distribution of plants in this mountain are the edaphic conditions, the local geomorphology, the intensity of land use but also anthropogenic activities such as grazing, bush fire and the cutting of wood. The Shannon diversity index obtained was high indicating a high diversity of species in this mountain.
The number of species recorded in the woody vegetation of the study is higher compared to the 33 species recorded by Konsala et al.19 in Mount Maroua (Far North Cameroon) and is small compared to the 80 species recorded by Nangndi et al.20 in the woody vegetation of Larmanaye (Chad). The differences observed in these studies could be attributed to the difference in the level of human influence through uncontrolled agriculture, cutting down of trees for firewood, overgrazing and bushfires.
Regarding families, Poaceae, Asteraceae and Fabaceae were the richest families in this study, this may be due to the high number of seeds produced by plants of these families. These families are also the most represented plant families in other studies in the Western Highlands region of Cameroon in the same order6,7,11. These results were different to those obtained by Konsala et al.19 in Mount Maroua, Far North Cameroon who found that Fabaceae and Poaceae were the richest families. The Poaceae are the fifth most diverse family of flowering plants and the second most diversified family of Monocotyledons (APG IV). The Asteraceae are the second largest family of flowering plants after Orchidaceae21. Zerbo et al.22 stated that the high presence of species of the Gramineae family is explained by the fact that savannahs are grass-dominated ecosystems. Moreover, Poaceae taxa have a high potential for tillage and a high regrowth rate after grazing if environmental conditions are favourable. According to Ramirez et al.23, the high proportion of Asteraceae can be attributed to their great ecological tolerance range and high seed dispersal capacity. Poaceae and Asteraceae species, due to their wide ecological range, are diverse in their habitat occurrence. The high number of families (48 families), which revealed a varied distribution of ora in the study area, could be explained by variation in microhabitat, morphological characteristics, life duration and dynamic ecological niche24.
The high value of the Shannon diversity index indicates the high diversity of the study area and can be associated with the high number of plant species and the diversity of the observed biotope (lowlands, slope area and road boundary). According to Baudoin et al.7, each topographic position corresponds to a specific type of soil and type of drainage, which can constitute niches for various plant species. The high evenness value means that the number of species recorded is in equilibrium.
The presence of threatened species/Vulnerable/Near-Threatened in Mount Mbapit, which is considered an ecologically fragile ecosystem subject to high anthropogenic activities (agricultural activities, grazing, bushfires and wood collection), suggests that this savannah ecosystem mountain is of considerable conservation importance. Judicious use of the available forest resources must be ensured by the government and measures taken to control human and animal exploitation of the mountain, to prevent its extinction in the coming decades and to make it available for future generations.
The dominance of this study sites by herbs corresponds to the previous studies which show that savannahs are ecosystems dominated by herbs6,10,11,19,23,25. The high proportion of Herbaceous species should be explained by the anthropogenic pressures (bushfires, overgrazing and firewood collection). Tropical savannahs are highly resilient and even dependent on frequent fires and mega-herbivores, which maintain savannah plant diversity and vegetation structure, i.e. low tree cover26.
The study of functional groups provides clear information on the physiological adaptations of plant communities to particular environmental conditions24. The predominance of phanerophytes, chamaephytes and therophytes over other life forms could be a response to the hot climate, topographic variations and anthropogenic pressure. Similar conclusions were also reported by Baudoin et al.6 and Mbogue et al.11 in their studies in the savannahs ecosystems. The phanerophytes of this study are mainly made up of trees and shrubs of savannah which are equipped with devices allowing them to resist the passage of current fires (thickening of the bark): Protea madiensis, Entada africana, Terminalia glaucescens and Vitellaria paradoxa are particularly demonstrative in this respect, these trees are never jointed. According to Baudoin et al.6 the plants of the regions which undergo bushfires with certain periodicity present a series of adaptations assuring survival or allowing fast colonization of the medium. These strategies include the capacity to reject strains, the existence of underground organs (bulbs and rhizomes), thick barks to resist high temperatures, the release of seeds, or the stimulation of their germinative capacities after bush burning27. The high representation of macrophanerophytes and mesophanerophytes could be explained by the fact that the vegetation cover of the Mbapit mountain was primarily covered by wooded savannah. The proportion of therophytes in this savannah indicates high biotic disturbance levels on the habitat via grazing and bushfires. Moreover, variations in topography and climatic characteristics signi cantly affect the existence and distribution of various plant species and life forms.
Concerning leaf size, the most frequent are mesophylls, microphylls and notophylls, this could be explained by the influenced of humidity, light and wind. Ohsawa16 obtained the same results in the intact zones in sub-tropical forests. These findings are similar to the results of Baudoin et al.6 and Mbogue et al.11 in the Western Highlands of Cameroon.
Sarcochores and sclerochores were the most abundant types of diaspores. The importance of sarcochores compared to other types of diaspores can be justified by the fact that these species are carried either by birds or by other animals and have the chance to arrive at their destination. Sclerochores or light non-fleshy diaspores, due to their lightness, are more likely to be dispersed by the wind. Similar results were obtained in the savannahs Highlands of Cameroon6 and, in the Nyungwe montane savannahs8 where, sclerochores were the main types of diaspores. The seed dispersal spectrum of the studied Mount Mbapit savannahs was characterized by the dominance of anemochory, followed by zoochory and autochory species. These results are consistent with those reported for other savannahs6,8,28. Anemochory is a principal strategy for diaspora dispersal in open canopy areas.
The high proportion of afro-tropical and pantropical species indicate disturbed areas29. The importance of species with broad phytogeographical amplitude translates to the loss of identity of the vegetation by the invasions of species with broad distribution. The high proportion of widely distributed taxa reflects the opening of this flora to external influences. This disturbance could be due to grazing and agricultural activities that strongly modify the original flora. Most of the pantropical species are annual weeds. These results are similar to previous investigations, African distribution species constitute a remarkable proportion of the flora studied6,27.
The creation of an integral ecological zone for the long-term preservation of present and future populations of threatened species and to restore the denuded areas in this mountain. A similar study should be made in the gallery forest and cultivated areas in order to have an exhaustive list of the species present.
CONCLUSION
A total of 144 species belonging to 110 genera and 50 families have been recorded in Mount Mbapit. It was revealed that the flora was dominated by Poaceae and Asteraceae families. Herbs were the most dominant growth habit. Anemochory is the main dispersal syndrome. Afro-tropical species were the most dominant chorotype. Sustainable management for restoration should be used as a practice of assisted forest regeneration of threatened species in the natural habitats of this mountain.
SIGNIFICANCE STATEMENT
This study examined the rich floristic potential and their ecological traits in Mount Mbapit. That was an important and necessary investigation to carry out because it has been noticed that species richness and diversity are under serious threats from anthropogenic pressures and climate change, especially in the mountain areas. Studying the overall ecological scenario and biodiversity might be helpful as a reference study for the protection and manageable utilization of plants.
ACKNOWLEDGMENT
The authors wish to acknowledge Idea Wild for the fieldwork materials support.
REFERENCES
- Onana, J.M., 2015. The world flora online 2020 project: Will Cameroon come up to the expectation? Rodriguésia, 66: 961-972.
- Burgess, N.D., A. Balmford, N.J. Cordeiro, J. Fjeldsa and W. Kuper et al., 2007. Correlations among species distributions, human density and human infrastructure across the high biodiversity tropical mountains of Africa. Biol. Conserv., 134: 164-177.
- Khan, W., S.M. Khan, H. Ahmad, A.A. Alqarawi, G.M. Shah, M. Hussain and E.F. Abd_Allah, 2018. Life forms, leaf size spectra, regeneration capacity and diversity of plant species grown in the Thandiani Forests, District Abbottabad, Khyber Pakhtunkhwa, Pakistan. Saudi J. Biol. Sci., 25: 94-100.
- Solefack, M.C.M., E.F. Fedoung and L.F. Temgoua, 2018. Factors determining floristic composition and functional diversity of plant communities of Mount Oku forests, Cameroon. J. Asia-Pac. Biodiversity, 11: 284-293.
- Altaf, A., S. Marifatul Haq, N. Shabnum and H.A. Jan, 2022. Comparative assessment of phyto diversity in Tangmarg Forest Division in Kashmir Himalaya, India. Acta Ecol. Sin., 42: 609-615.
- Baudoin, W.T.J., A.T.M. Louise, F. Moksia, H. Yougouda, C.N.N. Mbogue, N.V. Francois and F. Theophile, 2020. Savannas highlands of Cameroon: Floristic composition, functional traits and conservation status. Asian J. Res. Bot., 3: 403-421.
- Baudoin, W.T.J., N.V. Francois and F. Théophile, 2017. Floristic diversity of Western Highlands Savannas of Cameroon. Int. J. Curr. Res. Biosci. Plant Biol., 4: 7-13.
- Bizuru, E., P. Niyigaba and M. Mujawamariya, 2014. Phytosociological study of Nyungwe Montane Savannahs. J. Nat. Sci. Res., 4: 67-78.
- Wouokoue, T.J.B., G.M. Anjah, V.F. Nguetsop and T. Fonkou, 2017. Floristic diversity of the savannah ecosystems in three altitudinal zones of the Bambouto Mountains, West Cameroon. Cameroon J. Biol. Biochem. Sci., 25: 52-59.
- Baudoin, W.T.J., A.T.M. Louise, H. Yougouda, N.V. Francois, T. Roger and N.M. Jonathan, 2020. Floristic diversity and management of fodder resources of the natural pastures of the Savanna Highlands of Western Cameroon. J. Exp. Sci., 11: 28-34.
- Mbogue, C.N.N., A.M. Grace, W.T.J. Baudoin and N.F. Jong, 2020. Phytosociology of echinops giganteus in the Western Highland of Cameroon. Eur. Sci. J., 16: 345-360.
- Ngnignigniwou, J.M., J.B.W. Taffo and V.F. Nguetsop, 2022. Woody species diversity and ecological characteristics of the Mawouon Forest, in the Western Highlands of Cameroon. Cameroon J. Exp. Biol., 15: 28-34.
- Youga, M.K.D., P.S. Njiméli, C.P. Kenfack, J.B.W. Taffo, W.N. Tacham and T. Fonkou, 2022. Knowledge and traditional uses of some aromatic and cosmetic plants species in the Western Highlands of Cameroon. Open J. Appl. Sci., 12: 1698-1718.
- Ndonmou, E.C., J.B.T. Wouokoue, M.C. Tankou, C.H.S. Sime and M.L.T. Avana, 2022. Contribution of cocoa and coffee agroforests to the conservation of plant biodiversity in the humid savannahs of Western Cameroon [In French]. Cameroon J. Exp. Biol., 16: 67-75.
- Raunkiaer, C., 1934. The Life Forms of Plants and Statistical Plant Geography. Clarendon Press, Oxford, London, UK, Pages: 632.
- Ohsawa, M., 1995. Latitudinal comparison of altitudinal changes in forest structure, leaf-type, and species richness in humid monsoon Asia. Vegetatio, 121: 3-10.
- Dansereau, P. and K. Lems, 1957. The Grading of Dispersal Types in Plant Communities and Their Ecological Significance. Inst. Bot. de l'Univ., Paris, Pages: 52.
- Gonmadje, C.F., C. Doumenge, T.C.H. Sunderland, M.P.B. Balinga and B. Sonké, 2012. Phytogeographical analysis of Central African forests: The case of the Ngovayang Massif (Cameroon) [In French]. Plecevo, 145: 152-164.
- Konsala, S., J.B.W. Taffo, R.N. Douanla, M.L.A. Tientcheu and E.M. Tchinda, 2022. Plant diversity and ecological characteristics along an altitudinal gradient in the Mount Maroua, Far North Cameroon. Asian J. Biol. Sci., 15: 5-14.
- Nangndi, B., M.L.A. Tientcheu, J.B.W. Taffo, A.B.E. Dong, D.T. Wolwai, T. Fonkou, 2021. Floristic and structural diversity of woody vegetation in the Sudano-Guinean Zone of Larmanaye, Chad. J. Ecol. Nat. Environ., 13: 63-72.
- Kenicer, G., 2006. Legumes of the World. Edited by G. Lewis, B. Schrire, B. MacKinder & M. Lock. Royal Botanic Gardens, Kew. 2005. xiv + 577pp., colour photographs & line drawings. ISBN 1 900 34780 6. £55.00 (hardback). Edinburgh J. Bot., 62: 195-196
- Zerbo, I., M. Bernhardt-Römermann, O. Ouédraogo, K. Hahn and A. Thiombiano, 2016. Effects of climate and land use on Herbaceous species richness and vegetation composition in West African Savanna Ecosystems. J. Bot., 2016: 9523685.
- Ramírez, N., N. Dezzeo and N. Chacon, 2007. Floristic composition, plant species abundance and soil properties of Montane Savannas in the Gran Sabana, Venezuela. Flora, 202: 316-327.
- Marifatul Haq, S., A.A. Khoja, F.A. Lone, M. Waheed, R.W. Bussmann, E.A. Mahmoud and H.O. Elansary, 2023. Floristic composition, life history traits and phytogeographic distribution of forest vegetation in the Western Himalaya. Front. For. Global Change, 6.
- Amber, K., K.R. Khan, A.H. Shah, M.F. Lodhi, M. Hussain and G.M. Shah, 2019. A comprehensive survey of floristic diversity evaluating the role of institutional gardening in conservation of plant biodiversity. Int. J. Biosci., 14: 310-324.
- Archer, S., T.W. Boutton and K.A. Hibbard, 2001. Trees in Grasslands: Biogeochemical Consequences of Woody Plant Expansion. In: Global Biogeochemical Cycles in the Climate System, Schulze, E.D., M. Heimann, S. Harrison, E. Holland, J. Lloyd, I.C. Prentice and D. Schimel (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780126312607, pp: 115-137.
- Buisson, E., S. Le Stradic, F.A.O. Silveira, G. Durigan and G.E. Overbeck et al., 2019. Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands. Biol. Rev., 94: 590-609.
- Lazure, L. and J.S. Almeida-Cortez, 2006. Impact of neotropical mammals on seeds dispersal and predation. Neotrop. Biol. Conserv., 1: 51-61.
- Sinsin, B., 2001. Life forms and specific diversity of woodland associations in Northern Benin [In French]. Syst. Geog. Plants, 71: 873-888.
How to Cite this paper?
APA-7 Style
Junior Baudoin,
W.T., Flore,
N.J., Clovis,
K.O., Elodie,
M.T., Samuel Severin,
K.F., Jonathan,
N.M., Eric,
N.C., Alex Bleriot,
F.T., Marie Louise,
A.T., Theophile,
F., François,
N.V. (2023). Assessing Floristic Diversity and Ecological Characteristics of Mount Mbapit Savannah, Western Highlands of Cameroon. Asian Journal of Biological Sciences, 16(3), 351-365. https://doi.org/10.3923/ajbs.2023.351.365
ACS Style
Junior Baudoin,
W.T.; Flore,
N.J.; Clovis,
K.O.; Elodie,
M.T.; Samuel Severin,
K.F.; Jonathan,
N.M.; Eric,
N.C.; Alex Bleriot,
F.T.; Marie Louise,
A.T.; Theophile,
F.; François,
N.V. Assessing Floristic Diversity and Ecological Characteristics of Mount Mbapit Savannah, Western Highlands of Cameroon. Asian J. Biol. Sci 2023, 16, 351-365. https://doi.org/10.3923/ajbs.2023.351.365
AMA Style
Junior Baudoin
WT, Flore
NJ, Clovis
KO, Elodie
MT, Samuel Severin
KF, Jonathan
NM, Eric
NC, Alex Bleriot
FT, Marie Louise
AT, Theophile
F, François
NV. Assessing Floristic Diversity and Ecological Characteristics of Mount Mbapit Savannah, Western Highlands of Cameroon. Asian Journal of Biological Sciences. 2023; 16(3): 351-365. https://doi.org/10.3923/ajbs.2023.351.365
Chicago/Turabian Style
Junior Baudoin, Wouokoue, Taffo, Nnanga Jeanne Flore, Kengne Olivier Clovis, Mafouo Tchinda Elodie, Kenfack Feukeng Samuel Severin, Ngnignindiwou Mouncharou Jonathan, Ndonmou Cantona Eric, Fomekong Tane Alex Bleriot, Avana Tientcheu Marie Louise, Fonkou Theophile, and Nguetsop Victor François.
2023. "Assessing Floristic Diversity and Ecological Characteristics of Mount Mbapit Savannah, Western Highlands of Cameroon" Asian Journal of Biological Sciences 16, no. 3: 351-365. https://doi.org/10.3923/ajbs.2023.351.365

This work is licensed under a Creative Commons Attribution 4.0 International License.



