Use of Biomarkers in Crab (Callinectes sapidus, Rathbun 1896) as Pollution Indicators in a Niger Delta Creek
| Received 18 Apr, 2023 |
Accepted 05 Jul, 2023 |
Published 30 Sep, 2023 |
Background and Objective: Hydrocarbon extraction activities along the coastlines of the Niger Delta are rife for decades and have contributed to various categories of persistent pollutants in the aquatic ecosystem. The bioaccumulation and induction of some biochemical markers in the blue crab, Callinectes sapidus as indicators of chronic petrogenic pollutions was studied. Materials and Methods: In situ, measurements for water temperature, pH, electrical conductivity, salinity, total dissolved solids and dissolved oxygen were made at the sampling locations. Forty-eight female crabs (weight 149.2±0.02 g) were harvested with a 15 mm mesh size fishing net and taken to the laboratory in plastic containers filled with their habitat water for the estimation of biomarker levels. Results: Tissue accumulations of the Polynuclear Aromatic Hydrocarbons (PAHs) at OSD ranged from 0.02-0.09 μg g–1 and Mononuclear Aromatic Hydrocarbons (MAHs) from 0.01-0.02 μg g–1, but were 0.01 μg g–1 and undetected in tissues at ISD. The levels of Alanine Aminotransferase (ALT) (sig. = 0.032), Aspartate Aminotransferase (AST) (sig. = 0.045), Alkaline Phosphatase (ALP) (sig. = 0.007), total proteins (sig. = 0.036) and malondialdehyde (MDA) (sig. = 0.005) were all markedly higher at the OSD location at the 95% confidence limit. The ordination biplot revealed that the explained variations for axes 1 and 2 were 68.95 and 85.38% and the eigenvalues were 0.689 and 0.164, respectively. The multivariate pie classes showed that the largest sector for AST was OSD 2, MDA and ALT were OSD 1 and ALP was OSD 3. Elevated PAHs and MAHs appeared to induce the production of more ALP (r = 0.695 and 0.660, respectively, p<0.05) and PAHs alone induced the production of more proteins (r = 0.630 and p<0.05). Conclusion: It was confirmed that allocthonous input of petroleum pollutants in aquatic ecosystems could cause biological disruptions, including tissue bioaccumulation and other biochemical disruptions in proteins and enzyme activities.
| Copyright © 2023 Ogbuagu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Estuary is a partially enclosed body of water where freshwater and seawater meet and mix to establish brackish conditions. Therefore, in its natural state, the estuarine habitats are dynamic, with periodic and stochastic fluctuations in such attributes as water temperature, salinity, nutrients and oxygen levels.
Various anthropogenic activities in both types and magnitude could, however, modify these regimes. For example, hydrocarbon extraction activities along the coastlines of the Niger Delta are rife for decades and have contributed to various categories of persistent pollutants in the aquatic ecosystem of the area1-4. Other than the domestic category, industrial and ancillary activities have thus been noted as exerting significant physicochemical imbalances on the enmeshed biological communities of these estuarine ecosystems.
Of these industrial activities, oil and gas and their ancillaries are most probably the greatest contributors of pollutants in the delta coastlines of Nigeria, largely due to the huge crude oil reserve and their exploration and exploitation activities that have been ongoing for over 65 years. An example is the Port Harcourt Refining Company (PHRC) located in the Okrika Mainland, near Port Harcourt in Nigeria which is sited proximal to local coastline creeks, including the Ekerekana, Ogan and Ogu Creeks. Improperly treated oily effluents from the company are discharged directly into the Ekerekana Creek and concomitant to this, oily scums are seen floating on water surfaces and coating prop roots of aquatic flora growing in them and the neighboring other creeks. Additional inputs of the hydrocarbon pollutants are made by thriving illegal artisanal oil refining activities by local operators. Following these, local fisherfolks have reported oil-tainted fish and other seafood tissues caught from the creek5.
According to Whitehead6, coastline estuaries are noted as one of the most biologically productive ecological habitats on the planet and so their contaminations could exert various toxic effects on its biota. Unfavorable environmental conditions could impose suboptimal conditions to which the resident organisms must strive to compensate by resorting to various physiological adjustments. These adjustments have become valuable in biomonitoring and so are used as bioindicators of environmental stress and pollution6.
Of the biotopes utilized in aquatic monitoring, crustaceans have least been used, irrespective of their role in marine, estuarine and even freshwater habitats. Tsang et al.7, revealed that the Brachyura group is one of the most diverse crustaceans, with about 7,000 described species in 98 families with habitats ranging from marine to freshwater to terrestrial. Of the common crab species abundant in the Upper Bonny Estuary, within which the current study was conducted, is the blue crab, Callinectes sapidus. This species, which had been described as one of the most abundant estuarine macro-invertebrates along the Atlantic and Gulf Coasts of the USA, also serves as a commercially important food for inhabitants of the larger Niger Delta.
With increasing and indiscriminate environmental degradation of the delta areas on one hand and increasing population pressure on the other, the sustainable yield of aquatic food is threatened. Crustaceans are known to lack inducible defensive response mechanisms with a high degree of specificity and memory response as in their vertebrate counterparts8. Crustaceans only exhibit innate immune system responses as protection against potential pathogenic and other environmental invasions8,9. These and other responses constituting biomarkers have thus been employed to detect biochemical and tissue-level changes that indicate altered multiple biological and physiological measurements, including acute responses to contaminants10 and chronic stress11.
The current work therefore attempted to close a gap in knowledge of biomonitoring, by using bioaccumulation and biomarker levels in tissues of the blue crab (Callinectes sapidus) for the inference or establishment of pollution status of a coastline creek in the Upper Bonny estuary of the Niger Delta.
MATERIALS AND METHODS
The study was carried out between October, 2021 and November, 2022.
|
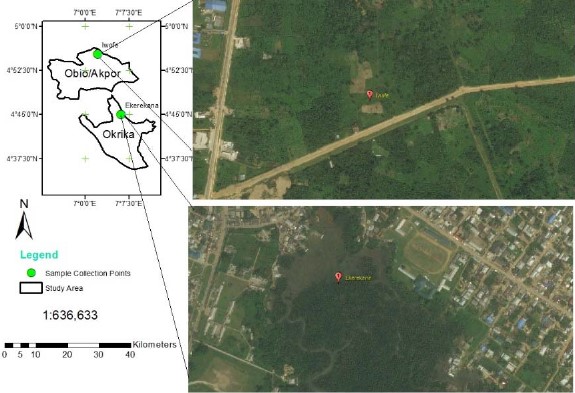
Study area: The Okrika Mainland in Okrika Local Government Area (LGA) and Iwofe in Obio-Akpor LGA of Rivers State, Nigeria (Fig. 1) are predominantly low-lying pluvial areas in the eastern part of the Niger Delta on the ocean-ward extension of the Benue Trough. The Niger Delta environment features several mangrove swamps, even as rainfall which is generally heavy between March and November is seasonal and variable, with a short dry season covering the rest of the year (December to February)12. Rainfall amounts of up to 2400-2600 mm are common, average temperatures are typically between 25 and 28°C and relative humidity rarely dips below 80% and fluctuates between 90 and 100% for most of the year. Oil exploration and production activities have been going on for over 65 years in the area and the major economic activities of inhabitants include fishing, farming, trading, artisanal labour and in a few cases civil service.
The Ekerekana Creek in Okrika Mainland is impacted by industrial effluent discharges from the proximal Port Harcourt Refining Company (PHRC), as well as from artisanal refining by local operators in its vicinity. The creek also serves for fisheries and as a transportation channel into other neighboring communities and the larger Bonny Estuary.
Field methods: Two sampling locations: The Ekerekana Creek (OSD) serving as the impacted location and Iwofe Waterside (ISD) serving as a reference location were studied. Three sampling points were selected within OSD and one within ISD. Each sampling point was geo-referenced with a Garmin GPmap 76 instrument.
In situ, the determination of water temperature, pH, electrical conductivity (EC), salinity, total dissolved solids (TDS) and dissolved oxygen (DO) was made with a pre-calibrated HANNA HI 9828 pH/ORP/EC/DO meter at each of the sampling points. Water samples for the determination of hydrocarbon concentrations were fixed with conc. H2SO4 in the ratio of 2:500 in 250 mL amber glass containers.
Sediment samples collected with 10×12 cm Eckman Grab were transferred into labeled polythene bags and transported to the laboratory.
A total of 48 female crab samples of approximately the same size and weight (149.2±0.02 g were harvested with a 15 mm mesh size fishing net and taken to the laboratory in plastic containers filled with their habitat water. The studies were approved by the Ethical Committee of the Federal University of Technology, Owerri where the work was performed.
Laboratory analysis
Analysis of hydrocarbons in sediment and water: The analytical procedure was in keeping with standard methods of APHA13 and Fetzer14. In sediments, the sample extraction procedure involved weighing out 5 g of a sediment sample into a beaker and adding 10 mL of analytical-grade hexane to the sample. The mixture was shaken for 5 min filtered and the filtrate was used for Gas Chromatography (GC) analysis.
For the water sample, 50 mL of a sample was put into a 1 L separating funnel. One drop of concentration H2SO4 was added to the sample in the separating funnel to release the hydrocarbon components and 5 mL of analytical grade N-hexane (used as solvent) was then added. Samples were thoroughly shaken for 5 min and allowed to stand for a further 20 min. Layers were formed that separated the top layer (extract) from the lower layer that was discarded. The extract was then collected in a glass vial for further analysis. Column chromatography (Agilent, USA, Model No: 5890) was set up with silica gel and glass wool and extracts were passed through the column for cleaning and removal of biogenic. They were then collected for GC analysis.
The GC was calibrated with commercially prepared external standards having 16 PAH components with a concentration of 1000 ppm per component. The GC parameters used were helium (as carrier gas), air and hydrogen (as fuel gases), nitrogen (as backup gas), detector temperature of 35°C, the in-let temperature of 25°C, initial oven temperature of 5°C, final oven temperature of 300°C, the hydrogen flow rate of 30 mL min–1, air flow rate of 300 mL min–1, nitrogen flow rate of 30 mL min–1 and helium flow rate of 30 mL min–1.
The GC parameters were set and a PAH extract was loaded using a micro-syringe to prompt the GC inter-phased with Flame Ionization Detector (GC-FID) (Agilent, USA, Model No: 5890) to run for a period of about 41 min.
Tissue accumulation analysis: Tissue samples were homogenized with NaSO4 for 2 or 3 min for adequate dryness, according to the method of Abdallah15. The mixture was placed in a pre-cleaned extraction thimble and the dehydrated tissue was extracted inside the thimble with 200 mL of n-hexane-dichloromethane in the ratio of 1:1 for 8 hrs in a Soxhlet apparatus, at a cycling rate of 5 or 6 times/hr. Anhydrous NaSO4 was extracted the same way as the sample was used as blank. The extracted solvents were concentrated down to 2 mL with a rotary evaporator at a maximum temperature of 35°C and then further concentrated down to 2 mL with a pure nitrogen gas stream. Clean-up and fractionation were conducted by passing the extract through a silica/alumina column. The first-milliliter volume of the extract was passed through slurry packing of 20 mL (10 g) silica, 10 mL (10 g) of aluminium and then 1 g of anhydrous NaSO4. Elution was made with 40 mL of hexane/dichloromethane in a ratio of 90:10 and then with 20 mL hexane/dichloromethane the ratio of 50:50. Eluted samples were then concentrated under a gentle stream of purified nitrogen to about 0.2 mL, before injection into a GC-FID (Agilent, USA, Model No: 5890).
Estimation of biomarkers: Only female crabs (identified with the presence of an egg pouch) were used for the analysis. Upon dissection, the ovaries and hepatopancreas were collected in sterile sample bottles, while the haemolymph was collected in heparinized bottles to prevent coagulation. The laboratory procedures were in keeping with the protocols of Osuagwu et al.16 and Vijayavel and Balasubramanian17, using kits provided by AGAPPE Diagnostics Ltd., India (www.agappe.com).
Alkaline Phosphatase (ALP): The principle derives from the activity of ALP in highly alkaline pH in the presence of divalent Mg ions where it catalysis the hydrolysis of p-Nitrolphenylphosphate (PNPP) which results in the release of p-Nitrophenol and a free phosphate group. Absorbance is proportional to the serum ALP at 405 nm. The enzyme ALP hydrolyses the 4-NPP to release 4-nitrophenol, under alkaline conditions. The 4-nitrophenol so formed is detected with a spectrophotometer at 405 nm to give a measurement of ALP activity in the sample:
The manufacturer’s kit components included the followings:
| • | Alkaline Phosphatase (S.L) Reagent 1 (R1), containing a mixture of 8.78 g L–1 HEDTA, 6 mL L–1 zinc sulphate, 5 g L–1 magnesium acetate and 92.6 AMP buffer | |
| • | Alkaline Phosphatase (S.L) Reagent 2 (R2), containing 50 mmol L–1 of P-Nitro phenyl phosphate |
The reagent was prepared by mixing 4 volumes of R1 with 1 volume of R2. The test parameters under kinetic mode and increasing slope of reaction included wavelength of 405 nm, temperature of 37°C, 2757 factor, D1 water as blank, linearity of 2000 U L–1, delay time of 60 sec, a sample volume of 20 μL, reagent volume of 1000 μL and 1 cm light path cuvette.
The AGAPPE Multicalibrator was used for the calibration of the auto-analyzer. Exactly 1000 μL of the working reagent was mixed with exactly 20 μL of hemolymph sample and incubated for I min at 37°C. The change in absorbance was measured per minute (OD/min) for 3 min. The ALP activity was calculated as follows:
Where, 2757 is a factor.
The AGAPPE Qualicheck Norm and Path (11601001) were used to verify the performance of the assay.
Lactate dehydrogenase (LDH): The principle for the kinetic determination of LDH is according to the reaction:
The reagent composition included:
| • | LDH-P (S.L) Reagent 1 (R1), containing a mixture of 80 mmol L–1 Tris buffer (at pH 7.4), 1.6 mmol L–1 pyruvate and 200 mmol L–1 NaCl | |
| • | LDH-P (S.L) Reagent 2 (R2), containing 240 mmol L–1 NADH |
Four volumes of R1 were mixed with 1 volume of R2. The decreasing slope of kinetic reaction parameters used was wavelength 340 nm, temperature 37°C, 16030 factor, D1 water blank, linearity 2400 U L–1, 60 sec delay time, 3 number of reading, 60 sec interval, sample volume 10 μL, reagent volume 1000 μL and 1 cm light path cuvette.
Exactly 1000 μL of the working reagent was mixed with 10 μL of ovaries and hepatopancreas extracts (extracted by crushing ovaries and hepatopancreas in normal saline solution, swirling and collecting supernatant) and incubated at 37°C for 1 min. The change in absorbance was measured per minute (OD/min) for 3 min. The LDP activity was calculated as follows:
Alanine Aminotransferase (ALT): The principle behind the kinetic determination of ALT is according to the reaction:
 |
The composition of the reagent is:
| • | SGPT (S.L) Reagent 1 (R1), containing a mixture of 110 mmol L–1 Tris buffer (pH 7.5), 600 mmol L–1 l-Alanine and >1500 U L–1 Lactate dehydrogenase (LDH) | |
| • | SGPT (S.L) Reagent 2 (R2), containing 16 mmol L–1 alpha-ketoglutarate and 0.24 mmol L–1 NADH |
Four volumes of R1 were mixed with 1 volume of R2. The decreasing slope kinetic reaction parameters used were 340 nm wavelength, 37°C temperature, 1745 factor, D1 water blank, 350 U L–1 linearity, 60 sec delay time, 3 number of readings, 60 sec interval, 100 μL sample volume, 1000 μL reagent volume and 1 cm light path cuvette.
One thousand μL of the working reagent was mixed with 100 μL of haemolymph sample and incubated for 1 min at 37°C. The change in absorbance was measured per minute (OD/min) for 3 min. The SGPT (ALT) activity was calculated as follows:
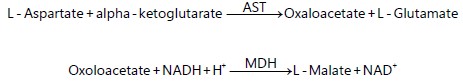
Aspartate Aminotransaminase (AST): The kinetic determination of AST is based on the following reaction:
 |
The reagent is composed of:
| • | SGPT (S.L) Reagent 1 (R1), containing a mixture of 88 mmol L–1 Tris buffer (pH 7.8), 260 mmol L–1 L-Aspartate, >1500 U L–1 Lactate dehydrogenase (LDH) and >900 U L–1 Malate dehydrogenase (MDH) | |
| • | SGPT (S.L) Reagent 2 (R2), containing 12 mmol L–1 alpha-ketoglutarate and 0.24 mmol L–1 NADH |
Four volumes of R1 were mixed with 1 volume of R2. The decreasing slope kinetic reaction parameters used were 340 nm wavelength, 37°C temperature, 1745 factor, D1 water blank, 350 U L–1 linearity, 60 sec delay time, 3 number of readings, 60 sec interval, 100 μL sample volume, 1000 μL reagent volume and 1 cm light path cuvette.
Exactly 1000 μL of the working reagent was mixed with 100 μL of haemolymph sample and incubated at 37°C for 1 min. The change in absorbance was measured per minute (OD/min) for 3 min. The SGOT (AST) activity was calculated as follows:
Malondialdehyde (MDA): The principle behind the estimation of serum/plasma (haemolymph) lipid peroxidation product malondialdehyde involves the reaction of Thiobarbituric acid (TBA) with MDA and end product lipid peroxidation, under the slightly acidic condition to produce a red trimetrone complex which absorbs maximally at 532 nm wavelength with a spectrophotometer (Varian 600 Spectra AA, USA). The equation for the reaction is as follows:
Exactly 0.25 mL of haemolymph and 1.25 mL of 10% trichloroacetic acid were added to a clean centrifuge tube and allowed for 10 min after this. Exactly 1.25 mL of 0.05 M H2SO4 and 1.5 mL of 0.67 TBA were added to the tube and mixed properly. The tubes were placed in a boiling water bath for 1 hr. They were then cooled under a running water tap and 2 mL of butan-1-ol was added. TBA reactive material was extracted and absorbance read at 532 nm wavelength. Values of the TBA-reactive material were extrapolated from the standard curve.
Total proteins: The colorimetric determination of total protein was based on the Biuret reaction (copper salt in an alkaline medium) principle. Protein in plasma or serum samples forms a blue-coloured complex when treated with cupric ions in an alkaline solution. The intensity of the blue colour is proportional to the concentration of protein.
The 2×50 mL Total Protein Reagent (TPR) is composed of 6 mmol L–1 potassium iodide, 21 mmol L–1 potassium sodium tartarate, 6 mmol L–1 copper sulphate and 58 mmol L–1 NaOH. The 1×3 mL Total Protein Standard (TPS) is composed of 6 g dL–1 TPS concentration.
The autoanalyzer systems parameters include the endpoint mode of reaction, the increasing slope of reaction, 546 nm wavelength, 37°C temperature, 6 g dL–1 standard concentration, 15 g dL–1 linearity, reagent blank, 10 min incubation time, 20 μL sample volume, 1000 μL reagent volume and 1 cm light path cuvette.
The reagent blank was mixed with 20 μL of haemolymph sample and 1000 μL reagent sample and incubated at 37°C. The absorbance of the standard and sample was measured against the reagent blank.
The total protein was calculated as follows:
Statistical analysis: With the SPSS v.22.0 software, possible homogeneity in the mean-variance of the biomarker levels was explored with the One-way ANOVA and their separation was achieved with a post-hoc Duncan’s Multiple Range Test at p<0.05. Pearson’s correlation coefficient (r) was used to explore the possible association of the inductor physicochemical attributes of the creek with biomarker levels. Redundancy Analysis (RDA) was used to further explore the spatial association of the biomarkers.
RESULTS
Pollutants in water and sediments: Water temperatures were between 30.33 at the ISD and 31.42 at OSD 3, pH was between 6.00 at OSD 2 and 6.68 at ISD, while Electrical conductivity (EC) was between 2608.00 μS/cm at ISD and 4912.00 μS/cm at OSD 3 locations (Table 1). Salinity, total dissolved solids (TDS) and dissolved oxygen (DO) varied from 434.67 0/00 at ISD to 818.67 0/00 at OSD 1, 1304.00 mg L–1 at ISD to 2456.00 mg L–1 at OSD 1 and 4.26 mg L–1 at OSD 1 to 4.72 mg L–1 at ISD locations respectively. Total polynuclear aromatic (=polyaromatic) hydrocarbons ( PAHs) varied from 0.07 at ISD to 10.23 mg L–1 at OSD 1. However, in sediments, the hydrocarbons ( PAHs and MAHs) varied from 6.20 and negligible mg/kg at ISD to 52.70 and 0.25 mg kg–1 at the OSD 3 locations. pH in sediments varied from 6.50 at OSD 2 and OSD 3 to 6.70 at the ISD locations.
Bioaccumulation of the hydrocarbons in tissues: At the impacted locations, PAHs ranged in the tissues from 0.02 μg g–1 (OSD 3) through 0.06 μg g–1 (OSD 2) to 0.09 μg g–1 (OSD 1), while MAHs ranged from 0.01 μg g–1 at OSD 1 and OSD 3 to 0.02 μg g–1 at OSD 2. However, at the reference ISD location, they were 0.01 μg g–1 and undetected in the tissues, respectively.
Concentrations of biomarkers in tissues: Table 2 shows the concentrations of the biomarkers measured in the blue crab across the sampling locations. Activities of lactate dehydrogenase (LDH) varied from 29.33±1.85 U L–1 at ISD to 42.33±6.35 U L–1 at OSD 1, alanine aminotransferase (ALT) varied from 24.03±1.35 U L–1 at ISD to 42.53±6.57 U L–1 at OSD 1, aspartate aminotransferase (AST) varied from 45.83±1.44 U L–1 at ISD to 66.97±7.34 U L–1 at OSD 2, alkaline phosphatase (ALP) varied from 12.93±0.33 at ISD to 21.33±0.60 U L–1 at OSD 3, total proteins varied from 3.80±0.45 g dL–1 at ISD to 5.60±0.25 g dL–1 at OSD 3, while malondialdehyde (MDA) varied from 13.00±1.15 mmol L–1 at ISD to 27.67±3.17 mmol L–1 at OSD 3 locations (Fig. 2).
The One-way Analysis of Variance (ANOVA) revealed that the physicochemical attributes of the creek’s water temperature, pH, EC, salinity, TDS and DO varied markedly (sig. = 0.000 each) across the sampling locations at p<0.05. However, PAHs and MAHs levels did not show significant spatial variability (sig. = 0.496 and = 0.427, respectively).
| Table 1: | Concentrations of hydrocarbons and some physicochemical attributes in water and sediments of the Ekerekana and Iwofe Creeks in the Niger Delta | |||
Sampling locations |
||||
| Coordinates | OSD 1 |
OSD 2 |
OSD 3 |
ISD |
| North | 04°44'20.4" |
04°44'2.4" |
04°44'38.4" |
04°55'4.8" |
| East | 07°06'39.6" |
07°06'54" |
07°05'20.4" |
07°01'15.6" |
Water |
||||
| Temperature (°C) | 31.2 |
31.33 |
31.42 |
30.33 |
| pH | 6.29 |
6 |
6.2 |
6.68 |
| EC (μS/cm) | 4912 |
3783.9 |
2988.55 |
2608 |
| Salinity (0/00) | 818.67 |
630.65 |
498.09 |
434.67 |
| TDS (mg L–1) | 2456 |
1891.95 |
1494.27 |
1304 |
| DO (mg L–1) | 4.26 |
4.56 |
4.68 |
4.72 |
| ∑PAHs (mg L–1) | 10.23 |
9.55 |
10.1 |
0.07 |
| ∑MAHs (mg L–1) | 0 |
0 |
0 |
0 |
Sediment |
||||
| pH | 6.6 |
6.5 |
6.5 |
6.7 |
| ∑PAHs (mg kg–1) | 37.85 |
51.67 |
52.7 |
6.2 |
| ∑MAHs (mg kg–1) | 0.18 |
0.22 |
0.25 |
0 |
| EC: Electrical conductivity, TDS: Total dissolved solids, DO: Dissolved oxygen, ∑PAHs: Total polynuclear aromatic hydrocarbons and ∑MAHs: Total mononuclear aromatic hydrocarbons (=benzene, toluene, ethylbenzene, xylene) | ||||

|
| Table 2: | Biomarker levels in tissues of the blue crab Callinectes sapidus from the Ekerekana and Iwofe Creeks of the Niger Delta | |||
Sampling locations |
||||||||||||
| Coordinates | OSD 1 |
OSD 2 |
OSD 3 |
ISD |
||||||||
| North | 04°44'20.4" |
04°44'2.4" |
04°44'38.4" |
04°55'4.8" |
||||||||
| East | 07°06'39.6" |
07°06'54" |
07°05'20.4" |
07°01'15.6" |
||||||||
| Biomarkers | 1 |
2 |
3 |
1 |
2 |
3 |
1 |
2 |
3 |
1 |
2 |
3 |
| LDH (U L–1) | 55 |
35 |
37 |
38 |
41 |
39 |
37 |
39 |
44 |
33 |
28 |
27 |
| ALT (U L–1) | 48.5 |
29.4 |
49.7 |
30 |
34 |
32.7 |
27.6 |
32.4 |
29.7 |
21.6 |
26.3 |
24.2 |
| AST (U L–1) | 59.4 |
56.2 |
64.3 |
81.4 |
57.4 |
62.1 |
54.6 |
67.4 |
60.3 |
48 |
46.4 |
43.1 |
| ALP (U L–1) | 18.6 |
21.1 |
14.6 |
15.4 |
19.8 |
17.8 |
21.3 |
22.4 |
20.3 |
12.7 |
13.6 |
12.5 |
| ∑Proteins (g dL–1) | 5.4 |
4.9 |
5.1 |
4.9 |
6.1 |
4.5 |
5.9 |
5.8 |
5.1 |
4.3 |
4.2 |
2.9 |
| MDA (mmol L–1) | 31 |
22 |
29 |
21 |
15 |
19 |
22 |
33 |
28 |
15 |
13 |
11 |
| LDH: Lactate dehydrogenase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase and MDA: Malondialdehyde | ||||||||||||
Of the biomarkers, the activities of ALT (sig. = 0.032), AST (sig. = 0.045) and ALP (sig. = 0.007) and the concentration of total proteins (sig. = 0.036) and MDA (sig. = 0.005) all differed markedly across the location at the 95% confidence limit. A post-hoc mean separation with the Duncan’s Multiple Range Tests revealed that though spatial differences in physicochemical attributes were in all the locations, that in the biomarkers were between OSD 1 = OSD 2 = OSD 3 and ISD (LDH, AST, ALP and Proteins), between OSD 1 = OSD 2 and OSD 3 = ISD (ALT) and between OSD 1 = OSD 3 and OSD 2 = ISD (MDA).
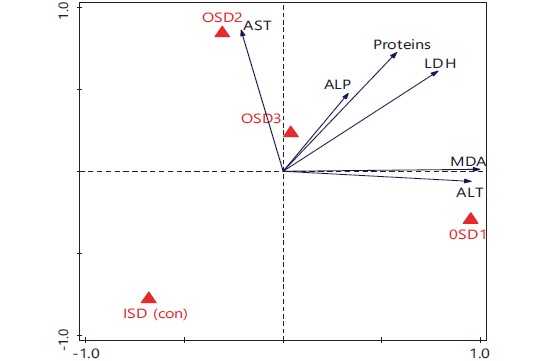
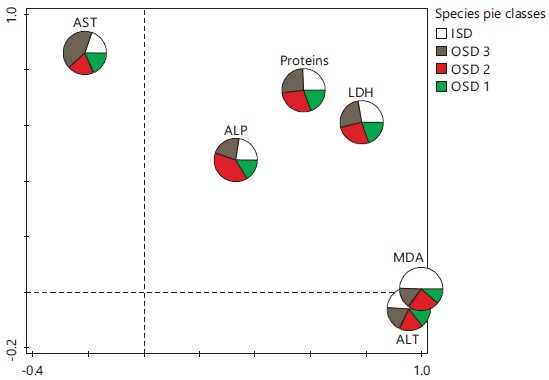
The ordination biplot output of multivariate redundancy analysis (Fig. 3) showed that the explained variations for axes 1 and 2 were 68.95 and 85.38% and the eigenvalues were 0.689 and 0.164, respectively. Inductions in ALP, LDH and proteins were highly associated with location OSD 3. On the contrary, those in MDA and ALT were highly associated with location OSD 1, while those in AST were dominant at location OSD 2. The control location (ISD) recorded the lowest inductions in all the biomarkers investigated. Correspondingly, the multivariate pie classes in Fig. 4 showed that the largest sector for AST was OSD 2. The MDA and ALT were mostly induced at location OSD 1, while OSD 3 was associated with the largest inductions for ALP. On the other hand, proteins and LDH were almost equally induced at all the locations.
The Pearson’s correlation (Table 3) revealed that water temperature correlated positively with LDH (r = 0.658, p<0.05), AST (r = 0.728), ALP (r = 0.813) and total proteins (r = 0.787, p<0.01). The pH correlated negatively with LDH (r = -0.593) and ALP (r = -0.655, p<0.05), AST (r = -0.831) and total proteins (r = -0.708, p<0.01), while EC correlated positively with ALT (r = 0.788, p<0.01). Salinity and TDS each correlated positively with ALT (r = 0.788 and 0.787 respectively) at p<0.01. Dissolved oxygen (DO) correlated negatively with ALT (r = -0.792, p<0.01), while total PAHs and MAHs correlated positively with ALP (r = 0.695 and 0.660, respectively at p<0.05 and total PAHs alone correlated with total proteins (r = 0.630, p<0.05).
|
|
| Table 3: | Correlation (r) matrix between the physicochemical attributes of the Ekerekana Creek and Iwofe waterside and biomarker levels in Callinectes sapidus | |||
| Temp | Ph | EC | Salinity | TDS | DO | PAHs | MAHs | |
| LDH | 0.658* | -0.593* | 0.536 | 0.536 | 0.536 | -0.488 | 0.201 | 0.176 |
| ALT | 0.451 | -0.391 | 0.788** | 0.788** | 0.787** | -0.792** | 0.122 | 0.143 |
| AST | 0.728** | -0.831** | 0.421 | 0.421 | 0.42 | -0.313 | 0.266 | 0.261 |
| ALP | 0.813** | -0.655* | 0.248 | 0.248 | 0.248 | -0.169 | 0.695* | 0.660* |
| Proteins | 0.787** | -0.708** | 0.342 | 0.342 | 0.342 | -0.260 | 0.630* | 0.501 |
| MDA | 0.680* | -0.509 | 0.473 | 0.473 | 0.473 | -0.471 | 0.284 | 0.342 |
| *Significant at p<0.05 level, **Significant at p<0.01 level, LDH: Lactate dehydrogenase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase and MDA: Malondialdehyde | ||||||||
DISCUSSION
The significantly higher hydrocarbon concentrations recorded in tissues of crab sampled from the impacted than reference locations indicate that effluents from the nearby PHRC which are discharged directly into the creek, as well as those from artisanal refining activities along the coastlines, contain petroleum pollutants.
Results from the current work support the knowledge that water bodies are the ultimate repository of wastes generated by industries and artisans5. The accumulation of the hydrocarbons in tissues of the organism sampled therefore came from allocthonous input of the pollutants from industrial and artisanal sources proximal to the creek. There exist both local18,19 and foreign research findings20,21 in support that contaminants are introduced into aquatic ecosystems via several routes and sources, including industrial, domestic and municipal run-offs and leachates. Alam et al.21 and Akan et al.22 also reported that effluents from various factory processes that are discharged untreated into nearby water bodies may affect water quality and also result in acute or chronic effects on resident organisms.
Hydrocarbons have been implicated in adverse health conditions such as cancer, respiratory disease, body fatigue, headache and other health conditions when they get incorporated into the food chain and are consumed by higher organisms23,24.
The concentrations in water and sediments and accumulations in tissues of the test organism in this work, as well as the outrageously high Hazard Quotient (HQ) values earlier reported by Ogbuagu et al.5 in a nearby Ogu Creek further affirm that the Niger Delta aquatic ecosystems are largely associated with petroleum hydrocarbon pollution. This high HQ value indicates high toxicity predisposition of consumers of this seafood and incidents of endocrine disruption and carcinogenicity from hydrocarbon pollutants have severally been indicated25-28.
Effluents from industrial activities have led to slightly elevated water temperature, as well as significant elevations in dissolved ions that constitute electrical conductivity, salinity and total dissolved solids in the impacted location. Moreover, PAHs and MAHs levels were markedly higher in the impacted location than in the reference location. Effluents from industrial production lines are often hot and contain dissolved inorganic ions. Artisanal and domestic sources may have exacerbated this input. These elevations are seen to have led to significant bioaccumulation of the hydrocarbon species measured in the tissues of the test organism. Bioconcentration and then the accumulation of stable and persistent pollutants will always occur whenever ambient levels in enmeshing media are higher than systemic levels in the organism.
These elevations in measured physicochemical variables appeared to have impacted the biomarker levels in the organism as well, as reflected by the correlation analysis. For example, slight acidity of water was associated with decreasing LDH, AST and ALP activities and concentrations of total proteins, while decreasing dissolved oxygen appeared to impact the activities of ALT. Elevated conductivities, salinity and dissolved solids appeared to have induced increased synthesis of ALT, with concomitant elevation of its activity, even as elevated PAHs and MAHs appeared to have induced increased synthesis of ALP and total proteins (PAHs only). It is known that the nutritive value of edible organisms, including crustaceans depends on their biochemical composition and that biochemical inductions by pollutants could distort systemic metabolism that could be manifested as inhibitions of enzyme activities and synthesis among other effects. For example, Vijayavel and Balasubramanian17 observed that even though the PAHs have their target sites of action in organisms, most of them depress metabolism, generally affecting biologically active molecules activities, including protein, nucleic acids, carbohydrates and lipids.
The induction of protein synthesis by PAHs and MAHs could indicate that when the organism was under hydrocarbons stress, it obtained extra energy from the tissue protein through the process of proteolysis, during which free amino acids used in the trichloroacetic acid cycle for energy production was produced in response. The elevation of LDH activity, a tetrameric enzyme recognized as a marker for assessing the toxicity of chemicals, in haemolymph could be probably due to the release of isozymes from the destroyed tissues17. LDH is also an important glycolytic enzyme inducible by oxygen stress29, similar to the findings in this work.
Alkaline phosphatase as phosphatases, are non-specific phosphomonoesterases with pH specificity that hydrolyses several phosphate esters and thus liberate phosphates from stored substrates of hepatopancreas during various physiological processes30. Phosphatases also play significant roles in the molting physiology of many crustaceans17. ALP is involved in the metabolism of carbohydrates, growth and differentiation, protein synthesis, synthesis of certain enzymes, secretion activity and phosphorylated intermediates transportation across cell membranes. Introduction by pollutants can, however, alter the phosphatase activity, similar to observations in the current work.
During protein synthesis, the mechanism involved in maintaining a balanced pool of free amino acids incorporates transamination which operates with two types of transaminases, i.e., alanine aminotransaminase and aspartate aminotransaminase. These transaminases are involved in the catalytic inter-conversion of amino acids and α-keto acids by the transfer of amino groups. The α-ketoglutarate/ L-glutarate couple serves as an amino group acceptor and donor pair in amino-transfer reactions. While ALT catalyzes the transfer of the amino group from alanine to α-ketoglutarate to form glutarate and pyruvate, AST, on the other hand, catalyses the transfer of the amino group from α-ketoglutarate to form glutarate and oxaloacetate31. Therefore, elevations in levels of these enzyme activities in extracellular fluid or plasma could be regarded as indicators of even minor cellular damages, caused in this case by pollutants in the organism’s enmeshing water environment. The change in ALT and AST activities could also be due to variations in protein metabolism in the tissues due to petroleum pollutant stress, an observation also made with naphthalene exposure of the edible estuarine crab, Scylla serrata by Vijayavel and Balasubramanian17.
When PAHs enter the body of organisms, systemic biotransformation processes cause their detoxification by converting the compounds into easily excretable products. During the detoxification process, reactive intermediates which are often more highly toxic than the parent compound are also synthesized. These intermediates bind covalently to the nucleophilic centers in such cellular macromolecules as DNA, RNA and proteins and so cause toxic reactions.
CONCLUSION
This study revealed that allocthonous input of pollutants from the petroleum industry in aquatic ecosystems caused biological disruptions, including tissue bioaccumulation and other biochemical disruptions in the proteins and enzyme activities of an aquatic organism. These disruptions in the resident organism could rightly infer pollution.
SIGNIFICANCE STATEMENT
The current work was an attempt to provide knowledge about biomonitoring, by using bioaccumulation and biomarker levels in tissues of the blue crab (Callinectes sapidus) to infer or establish the pollution status of a coastline creek in the Upper Bonny Estuary of the Niger Delta. The study showed elevated
petroleum hydrocarbon pollution in both water and tissues of the test organism, as well as biochemical disruptions in protein and enzyme activities. It calls for public health concerns among consumers of seafood in the area.
ACKNOWLEDGMENT
We acknowledge the assistance of Dr. E.T. Adebayo of the Department of Fisheries and Aquaculture Technology, Federal University of Technology, Owerri in the identification of the test organism.
REFERENCES
- Clinton, H.I., G.U. Ujagwung and M. Horsfall, 2009. Evaluation of total hydrocarbon levels in some aquatic media in an oil polluted mangrove wetland in the Niger Delta. Appl. Ecol. Environ. Res., 7: 111-120.
- Ogbuagu, D.H., E.T. Adebayo and E.I. Iwuchukwu, 2014. The role of oxygen in degradation of hydrocarbons in sediments of an estuary in Nigeria. J. Water Resour. Ocean Sci., 3: 45-50.
- Moslen, M. and A. Aigberua, 2018. Heavy metals and hydrocarbon contamination of surface water in Azuabie creek within Bonny estuary, Nigeria. J. Appl. Sci. Environ. Manage., 22: 1083-1088.
- Anaero-Nweke, G.N., A.P. Ugbomeh, I.K.E. Ekweozor, M. Molsen and N. Ebere, 2018. Heavy metal levels in water, sediment and tissues of Sarotherodon melanotheron from the Upper Bonny Estuary, Nigeria and their human health implications. Int. J. Mar. Sci., 8: 186-194.
- Ogbuagu, D.H., M.O. Idewele and K.P. Nzekwue, 2019. Bioaccumulation of persistent environmental pollutants (PEPs) in the mud catfishes (Clarias gariepinus and Heterobranchus longifilis) from the Ogu Creek, Rivers State, Nigeria. Int. J. Mar. Sci., 9: 54-65.
- Whitehead, A., 2013. Interactions between oil-spill pollutants and natural stressors can compound ecotoxicological effects. Integr. Comp. Biol., 53: 635-647.
- Tsang, L.M., C.D. Schubart, S.T. Ahyong, J.C.Y. Lai and E.Y. Au et al., 2014. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol. Biol. Evol., 31: 1173-1187.
- Söderhäll, K. and L. Cerenius, 1992. Crustacean immunity. Annu. Rev. Fish Dis., 2: 3-23.
- Roch, P., 1999. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture, 172: 125-145.
- Broeg, K., H.V. Westernhagen, S. Zander, W. Körting and A. Koehler, 2005. The “bioeffect assessment index” (BAI): A concept for the quantification of effects of marine pollution by an integrated biomarker approach. Mar. Pollut. Bull., 50: 495-503.
- Hyne, R.V. and W.A. Maher, 2003. Invertebrate biomarkers: Links to toxicosis that predict population decline. Ecotoxicol. Environ. Saf., 54: 366-374.
- Osuji, L.C. and U.C. Opiah, 2007. Hydrocarbon contamination of a terrestrial ecosystem: The case of Oshire-2 oil spill in Niger Delta, Nigeria. Environmentalist, 27: 337-340.
- APHA, 1998. Standard Methods for the Examination of Water and Wastewater. 20th Edn., American Public Health Association, Washington, D.C., ISBN: 9780875532356.
- Fetzer, J.C., 2007. The chemistry and analysis of large PAHs. Polycyclic Aromat. Compd., 27: 143-162.
- Abdallah, M.A.M., 2017. Bioaccumulation of hydrocarbons in freshwater fish species cultured in a shallow Coastal Lagoon, Egypt. Earth Syst. Environ., 1: 2.
- Osuagwu, U.O., C.O. Ujowundu, L.A. Nwaogu and R.N. Nwaoguikpe, 2023. Biochemical and oxidative stress responses in Clarias gariepinus exposed to sublethal concentrations of benzo[a]pyrene. Asian J. Biochem. Genet. Mol. Biol., 13: 15-22.
- Vijayavel, K. and M.P. Balasubramanian, 2006. Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in the edible estuarine crab Scylla serrata. Ecotoxicol. Environ. Saf., 63: 141-147.
- Olowu, R.A., O.O. Ayejuyo, A. Adejoro, G.O. Adewuyi and M.O. Osundiya et al., 2010. Determination of heavy metals in crab and prawn in Ojo Rivers Lagos, Nigeria. Electron. J. Chem., 7: 526-530.
- Wangboje, O.M. and A.J. Ikhuabe, 2015. Heavy metal content in fish and water from River Niger at Agenebode, Edo State, Nigeria. Afr. J. Environ. Sci. Technol., 9: 210-217.
- Ikem, A., N.O. Egiebog and K. Nyavor, 2003. Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut., 149: 51-75.
- Alam, L., C.A.R. Mohamed and M.B. Mokhtar, 2012. Accumulation pattern of heavy metals in marine organisms collected from a coal burning power plant area of Malacca Strait. ScienceAsia, 38: 331-339.
- Akan, J.C., S. Mohmoud, B.S. Yikala and V.O. Ogugbuaja, 2012. Bioaccumulation of some heavy metals in fish samples from River Benue in Vinikilang, Adamawa State, Nigeria. Am. J. Anal. Chem., 3: 727-736.
- Copat, C., F. Bella, M. Castaing, R. Fallico, S. Sciacca and M. Ferrante, 2012. Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bull. Environ. Contam. Toxicol., 88: 78-83.
- Ujowundu, C.O., J.U. Ogbede, K.O. Igwe, G.N. Okwu, N.C. Agha and R.I. Okechukwu, 2015. Quantitative assessment of polycyclic aromatic hydrocarbons and heavy metals in fish roasted with firewood, waste tyres and polyethylene materials. Biochem. Anal. Biochem., 4: 1.
- Pasquevich, M.Y., M.S. Dreon, J.N.G. Rivera, C.V. Boucard and H. Heras, 2013. Effect of crude oil petroleum hydrocarbons on protein expression of the prawn Macrobrachium borellii. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol., 157: 390-396.
- Brooks, B.W., C.M. Foran, S.M. Richards, J. Weston and P.K. Turner et al., 2003. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett., 142: 169-183.
- Giri, S. and A.K. Singh, 2014. Assessment of human health risk for heavy metals in fish and shrimp collected from Subarnarekha River, India. Int. J. Environ. Health Res., 24: 429-449.
- Ujowundu, C.O., C.O. Ajoku, L.A. Nwaogu, D.C. Belonwu and K.O. Igwe, 2014. Toxicological impact of gas flaring and other petroleum production activities in the Niger Delta area of Nigeria. J. Adv. Chem., 10: 2297-2304.
- Mishra, R. and S.P. Shukla, 2003. Endosulfan effects on muscle malate dehydrogenase of the freshwater catfish Clarias batrachus. Ecotoxicol. Environ. Saf., 56: 425-433.
- Zhou, X.W., Q.X. Chen, Z. Chen, Z.Q. He and H.M. Zhou, 2000. Effects of oxodiperoxovanadate (V) complexes on the activity of green crab (Scylla serrata) alkaline phosphatase. Biochemistry (Moscow), 65: 1424-1428.
- Burtis, C.A., E.R. Ashwood and N.W. Tietz, 1999. Tietz Textbook of Clinical Chemistry. 3rd Edn., W.B. Saunders, Philadelphia, ISBN: 9780721656106, Pages: 1917.
How to Cite this paper?
APA-7 Style
Ogbuagu,
D.H., Obinna,
D.U., Ujowundu,
C.O., Ibeh,
O.O. (2023). Use of Biomarkers in Crab (Callinectes sapidus, Rathbun 1896) as Pollution Indicators in a Niger Delta Creek. Asian Journal of Biological Sciences, 16(3), 240-253. https://doi.org/10.3923/ajbs.2023.240.253
ACS Style
Ogbuagu,
D.H.; Obinna,
D.U.; Ujowundu,
C.O.; Ibeh,
O.O. Use of Biomarkers in Crab (Callinectes sapidus, Rathbun 1896) as Pollution Indicators in a Niger Delta Creek. Asian J. Biol. Sci 2023, 16, 240-253. https://doi.org/10.3923/ajbs.2023.240.253
AMA Style
Ogbuagu
DH, Obinna
DU, Ujowundu
CO, Ibeh
OO. Use of Biomarkers in Crab (Callinectes sapidus, Rathbun 1896) as Pollution Indicators in a Niger Delta Creek. Asian Journal of Biological Sciences. 2023; 16(3): 240-253. https://doi.org/10.3923/ajbs.2023.240.253
Chicago/Turabian Style
Ogbuagu, Dike, Henry, Doris Ugochi Obinna, Cosmas Onyekachi Ujowundu, and Olusola Olubunmi Ibeh.
2023. "Use of Biomarkers in Crab (Callinectes sapidus, Rathbun 1896) as Pollution Indicators in a Niger Delta Creek" Asian Journal of Biological Sciences 16, no. 3: 240-253. https://doi.org/10.3923/ajbs.2023.240.253

This work is licensed under a Creative Commons Attribution 4.0 International License.



