The Use of Ficus exasperata Extracts for the Treatment of Coccidiosis in Poultry
| Received 23 Jul, 2023 |
Accepted 30 Oct, 2023 |
Published 31 Dec, 2023 |
Background and Objective: Bioactive ingredients of Ficus exasperata extracts were found at different concentrations to be effective for the treatment of coccidiosis in broiler chickens infected with Eimeria tenella. The effect of Ficus exasperata in experimentally infected broiler chickens was carried out together with extraction of bioactive ingredients, anticoccidial activities and haematological variables was also determined. Materials and Methods: Broiler chickens used for the research work were divided into four groups A, B, C and D. All the birds were challenged per oral administration with 1 mL of a mixed suspension of sporulated oocysts of Eimeria tenella at two different concentrations and later treated with Ficus exasperata extracts. This was studied using different variances for a number of oocyst counts. Results: The oocyst counts after post-challenge showed that coccidiosis infection was established in each of the groups within 3-5 days. There was a significant reduction in the number of oocysts shedding per bird in all groups treated with Ficus exasperata. Hematological variables of groups infected and treated with Ficus exasperata did not significantly differ (p<0.05) from the group treated with the standard coccidial drug. Conclusion: The research was to determine the efficacy and anticoccidial activities of Ficus exasperata plant extracts in broiler chickens infected with Eimeria tenella and to serve as an alternative to the drugs used in the poultry industry.
| Copyright © 2023 Kolawole Ilesanmi Henry. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Chicken coccidiosis is an infection of the intestinal tract caused by a single-cell protozoan parasite according to literature. All livestock species, as well as wild animals, can be infected and it is especially prevalent when animals or birds are grouped in significant numbers1. Each species of livestock has a species-specific coccidian that causes infections in that species. Generally, coccidiosis occurs more frequently during warmer (May to September) than colder months (October to April) of the year. Eimeria acervulina and Eimeria maxima develop in epithelial cells within the small intestine and generally cause chronic intestinal coccidiosis2. There is no cross-infection between species, stages of coccidian in chickens appear both within the host and as well as outside, the developmental stages in the chicken give rise to a microscopic egg (oocysts) that is passed out in the droppings. Under proper conditions of temperature and moisture, the oocyst develops within one to two days to form a sporulated oocyst, which confers sensitivity on species of Eimeria tenella which is capable of infecting other chickens3. At this stage, the oocyst contains eight bodies (called sporozoites), each of which is capable of entering a cell in the chicken’s intestine after the oocyst is eaten4. When sporozoites enter the cells, they divide many times producing many offspring (merozoites). The numbers produced depend on the species of coccidian involved, each merozoite, in turn, may enter another intestinal cell5. Several factors influence the severity of infection. Some of these factors include a number of oocysts consumed. Generally, an increase in the number of oocysts eaten is accompanied by an increase in the severity of the disease. Studies from Fayer6 revealed that strains of coccidian play a vital role, different strains of a species may vary in pathogenicity, other factors include environmental factors affecting the survival of the oocysts, site of development within the host, though coccidian that develops superficially are less pathogenic than those that develop deeper6. Young birds are generally more susceptible than older birds but poorly fed birds are more susceptible7. Coccidiosis in chickens is classified as either intestinal or cecal, most serious cases of intestinal coccidiosis are caused by Eimeria necatrix, while cecal coccidiosis is caused by Eimeria tenella8. Coccidiosis occurs most frequently in young birds; older birds are generally immune as a result of prior infection. Severe damage to the ceca and small intestine accompanies the development of the coccidian, broilers and layers are more commonly infected, but broiler breeders are more susceptible8. The most easily recognized clinical signs of severe cecal coccidiosis are the presence of bloody droppings8. Dehydration may accompany cecal coccidiosis, this can be compared to the dietary modulation of the human colonic microbiota and birds9. Coccidiosis caused by E. tenella first becomes noticeable about three days after infection. The greatest amount of blood appears by day five or six and by the eighth or ninth day, the birds are either dead or on the way to recovery.
Mortality is highest between the fourth and sixth days. Death may occur unexpectedly, owing to excessive blood loss and birds that recover may develop a chronic illness as a result of persistent cecal damage10. Birds heavily infected with Eimeria necatrix may die before any marked change is noticed in weight or before blood is found in the faeces. Eimeria acervulina is less pathogenic than Eimeria tenella or Eimeria necatrix. Eimeria acervulina is responsible for sub-acute or chronic intestinal coccidiosis in broiler chickens11. The clinical signs include loss of weight and watery and whitish diarrhoea. Eimeria maxima produces few marked changes in the small intestine until the 5th day after infection12. After that time of severe infection, numerous small haemorrhages occur along with a marked production of thick mucus, the inner surface inflamed and the intestinal content consists of a pinkish mucoid secretion13. Studies from Jadhav et al.14 opined that good management practices help in controlling coccidiosis, anticoccidial drugs mixed with the feed are used to limit high levels of infection, broiler feeds and water should be kept away from droppings so as to prevent broiler chickens from coccidiosis. The study was to determine the efficacy and anticoccidial activities of the plant extracts in broiler chickens infected with coccidiosis in the poultry industry. The main objective of the research work is to find methods of eliminating resistance to drugs caused by some strains of protozoans and for the extracts to serve as alternatives for standard drugs used in poultry industries. It is also to determine the effects of experimentally-infected broiler chickens; evaluate the haematological and clinical responses of broiler chickens treated with the plant extracts and study the weight and histopathology of ceacum and duodenum in experimentally infected broiler chickens.
MATERIALS AND METHODS
Study area: The study was carried out in the Microbiology Department, Federal University of Technology, Akure, Ondo State, Nigeria from May, 2016 to September, 2016 with support from the Department of Animal Production and Health, Federal University of Technology, Akure, Ondo State, Nigeria.
Collection of plant materials and processing: The plant used for this research work was five hundred grams (500 g) of the plant powder (Ficus exasperata) (leaves). Fresh leaves of Ficus exasperata were obtained from the forest beside the School of Agriculture Annex, Federal University of Technology, Akure. The plant was properly authenticated at the herbarium of the Forestry Department of the Federal University of Technology, Akure. Fresh leaves of Ficus exasperata were air-dried for 2 weeks and pulverized into a fine powder using an electrical blending machine of the Department of Animal Production and Health, Federal University of Technology, Akure. The machine was manufactured by J&J Electrical Company, 2010 model, Oluyole Industrial Estate Ibadan, Oyo State, Nigeria.
Extraction of bioactive ingredients: Five hundred grams of the plant powder (Ficus exasperata) were separately measured and soaked in 500 mL of 95% ethanol for 72 hrs, this was carried out in an air-tight container to avoid moisture15. Filtration was done by using a sieving cloth to filter the solvent containing the bio-active ingredients of the filtrate and the filtrates obtained were concentrated by rotary evaporator, manufactured by D&G Electrical Industry, Oluyole Industrial Estate, Ibadan, Oyo State Nigeria. The concentrated filtrates obtained were preserved in a refrigerator and later used as anti-coccidial agents.
Experimental birds: The broiler chickens used for this research work were purchased from Obasanjo Farm in Ota, Ogun State. Broiler chickens were divided into four groups A, B, C and D. Chickens were fed with a normal commercial diet (vital feed). One-week pre-experimental brooding period was allowed during which chickens were administered glucose syrup as anti-stress16. Weight was respectively taken and recorded for each group on a weekly basis17.
Experimental design: A total of 90 broiler chickens were used for the research work, they were divided into three groups A, B and C. Each group was subdivided into three replicates wherein each replicate contained ten broiler chickens; they were reared for 35 days (5 weeks) before they were challenged with Eimeria tenella oocysts18. Group A was infected and later treated with Ficus exasperata extract while, group B was infected and treated with standard coccidial drug (positive control) and group C was infected and left untreated (negative control) Two different concentrations of Ficus exasperata extracts (20 and 40 mL L–1) were administered during the treatment of coccidial infection19.
Preparation of coccidial innoculum and subjecting birds to coccidial oocysts: The infected broiler chicken from the Teaching and Research farm of the Federal University of Technology, Akure was properly dissected by a Technologist, the intestinal contents were removed and tested by subjecting to microscopic examination to determine the oocyst count. The oocysts obtained from the infected broiler chickens were therefore dissolved in one litre of water and mixed with 2% potassium dichromate solution using the procedure described by Ruff20. On day 35 (5 weeks), experimental birds were directly challenged in each of the groups by oral administration with 1 mL of a mixed suspension of freshly sporulated oocysts of Eimeria tenalla (30,000 per mL) as described by Shirley et al.21. Fecal and litter samples were collected on a weekly basis for post-infection to determine oocysts per gram of samples by McMaster counting chamber technique as described by Vermeulen et al.22.
Determination of haematological variables: One broiler chicken was sacrificed and dissected from each group on 48 days (2 weeks post-challenge). Blood samples were collected into EDTA bottles for the serum test. Capillary tubes were used to collect blood for determination of Packed Cell Volume (PCV), this was properly done in the centrifuge under high-speed revolution for 5 min. Erythrocyte Sedimentation Rate (ESR), Red Blood Cells (RBC), haemoglobin, neutrophil, monophil, basophil, eosinophil and other haematological parameters were carried out.
Histopathological examination of internal organs: After proper dissection of the broiler chickens, the internal organs were removed, ceacum and duodenum were separated. The ceacum and duodenum were collected into small bottles, formalin was added for preservation and a histopathology examination of ceacum and duodenum was carried out in each group to know the level of tissue damage and the effect of plant extract administered.
Ethical consideration: The statement of animal rights was obtained from the Department of Animal Production and Health of the Federal University of Technology Akure, Ondo State, Nigeria before embarking on the research work and full permission was granted for the research to be done according to ethics and guidelines.
Statistical analysis: The statistical analysis was carefully carried out using Analysis of Variance (ANOVA) and SPSS at a 95% confidence interval with a 0.05 significant level.
RESULTS
The results of the comparative weight profile at week 1, there was a weight increase among the groups although the weight increase was highest in group 3. Also, at week 2 and week 3, there was an increase in body weight among the groups but appreciable weight gain was more noticed in week 4 are shown in Table 1.
The result of Table 1 showed that there was progressive and appreciable weight gain from week 1 to week 4 which ranges from 0.25 to 0.95 kg from groups 1 to 4 within 4 weeks.
The result of oocyst counts after five days’ post-infection was shown in Table 2; the result showed that coccidiosis infection was established in each of the groups within 3-5 days, due to the high number of the oocysts which the broiler chickens were shedding during this period. However, group 4 recorded the highest number of oocysts count. This was accompanied by a reduction in body weight, low and sluggish activities of the broiler chickens and bloody diarrhea. Although the sign was also present in the other groups i.e., Ficus 20 mL L–1 of water, Ficus 40 mL L–1 of water and positive control the mean oocyst count was relatively high. The result of oocyst counts after 2 weeks post-infection test as shown in the Table revealed that there was a significant reduction in the number of oocysts shedding per bird in all groups treated with the plant extracts. During this period signs of improvement were seen especially in the group treated with 40 mL L–1 of Ficus exasperata extracts but the group that was infected and not treated (negative control) recorded the highest number of oocysts followed by the group treated with 20 mL L–1 of Ficus exasperata. However, the group treated with anticoccidial drug (positive control) recorded a decrease in the number of oocyst shedding. The effect of Ficus exasperata extracts on the fecal oocyst after 2 weeks post-challenge showed that at Ficus 20 mL L–1 of water the oocyst count showed a slight decrease, while at Ficus 40 mL L–1 of water appreciable decrease in the number of oocyst counts was recorded. Group 3 (positive control) showed a significant decrease in oocyst count due to the effect of anticoccidial drug used but negative control recorded the highest numerical oocyst count due to no treatment given to this group.
| Table 1: | Comparative weight profile of broiler chicken treatment with extracts | |||
| Treatment | Week 1 |
Week 2 |
Week 3 |
Week 4 |
| T1 | 0.25±0.002a |
0.37±0.003a |
0.55±0.003a |
0.95±0.003a |
| T2 | 0.26±0.002b |
0.38±0.003a |
0.56±0.003a |
0.95±0.003a |
| T3 | 0.27±0.013a |
0.37±0.003a |
0.56±0.005a |
0.96±0.003a |
| T4 | 0.25±0.012a |
0.36±0.003a |
0.56±0.004a |
0.95±0.004a |
| Mean±SD (n = 3), Means with the same superscript letters along the same column are not significantly different (p>0.05), T1: Group 1, T2: Group 2, T3: Group 3 and T4: Group 4 | ||||
| Table 2: | Post infection count of oocyst after 5 days | |||
| Treatment | Oocysts |
| Group1 | 1360.00±177.76a |
| Group 2 | 1466.67±88.19a |
| Group 3 | 1306.67±236.74a |
| Group 4 | 1600.00±57.74a |
| 2 weeks post infection | |
| Group 1 | 673.33±99.555b |
| Group 2 | 426.67±17.638b |
| Group 3 | 126.67±17.638a |
| Group 4 | 2400.00±166.533d |
| Mean±SD (n = 3), Means with the same superscript letters along the same column are not significantly different (p>0.05), Group 1: Ficus 20 mL L–1 of water, Group 2: Ficus 40 mL L–1 of water, Group 3: Control 1 (infected and treated with anticoccidial drug) and Group 4: Control 2 (infected not treated) | |
| Table 3: | Haematological variables after infection and treatment | |||
| TMT | ESR |
PCV |
RBC |
HB |
LYM |
NEU |
MONO |
BAS |
| T1 | 1.67±0.33a |
31.00±2.0a |
206.33±18.89a |
10.33±0.63a |
63.33±1.20ab |
23.33±1.76a |
10.00±1.00a |
2.67±0.67a |
| T2 | 3.33±0.33b |
27.33±0.88a |
177.33±10.41a |
9.10±0.27a |
63.00±1.00a |
23.00±2.52a |
10.33±1.45a |
2.33±0.33a |
| T3 | 2.33±0.88ab |
28.33±2.40a |
183.67±22.78a |
9.83±0.59a |
64.33±1.76ab |
22.00±2.52a |
10.00±1.53a |
2.67±0.3ab |
| T4 | 2.33±0.33ab |
29.00±1.00a |
192.67±10.79a |
9.77±0.29a |
62.67±0.33a |
21.33±1.20a |
12.67±1.20a |
2.00±0.00a |
| T5 | 2.33±0.33ab |
29.00±1.53a |
192.00±16.09a |
9.67±0.49a |
63.33±0.88ab |
22.00±1.00a |
09.67±0.88a |
3.67±0.33b |
| T6 | 2.33±0.33ab |
27.67±0.67a |
183.67±4.26a |
9.30±0.15a |
67.00±1.16b |
18.33±1.20a |
11.67±1.45a |
2.33±0.33a |
| Mean±SD (n = 3), Means with the same superscript letters along the same column are not significantly different (p>0.05), TMT: Treatment, Group 1: Ficus 20 mL L–1 of water, Group 2: Ficus 40 mL L–1 of water, Group 3: Control 1 (infected and treated with anticoccidial drug), Group 4: Control 2 (infected not treated), ESR: Erythrocyte Sedimentation Rate, PCV: Packed Cell Volume, RBC: Red Blood Cell, HB: Haemoglobin, LYM: Lymphocyte, NEU: Neutrophil, MONO: Monophil, BAS: Basophil and EOS: Eosinophil | ||||||||
| Table 4: | Clinical manifestation and case mortality of birds challenge with sporulated oocysts of Eimeria tenella | |||
| Treatment | Group |
Infected birds |
Pre-patent period |
Clinical signs |
Case mortality |
| 40 mL extract | C1 |
5 |
3-7 days |
Ruffled feather |
1 |
C2 |
5 |
3-7 days |
Mild diarrhea |
- |
|
C3 |
5 |
3-7 days |
2 |
||
| 20 mL extract | D1 |
5 |
3-7 days |
Anorexia |
2 |
D2 |
5 |
3-7 days |
Mucoid diarrhea |
1 |
|
D3 |
5 |
3-7 days |
2 |
||
| +ve control | E |
5 |
3-7 days |
Normal body activities |
- |
| -ve control | F |
5 |
3-7 days |
Bloody droppings |
3 |
| Mean±SD (n = 3), C1 to C3: Groups infected and treated with 40 mL of Ficus exasperata, D1 to D3: Groups infected and treated with 20 mL of Ficus exasperata extract, E: Positive control infected and treated with standard drug and F: Negative control infected but not treated | |||||
Haematological test was properly carried out for the broiler chickens in all groups and the result of the haematological variables after 2 weeks post-infection as shown in Table 3. The results of haematological variables of groups infected and treated with the plant extract did not significantly differ (p<0.05) from the group treated with the standard coccidial drug. This reduction might result from the possible migration of lymphocytes into tissue in response to the infection from pathogenic organisms. There was a difference in haematological parameters compared to infected not treated (negative control) which showed loss of weight and an increase in the amount of mucoid diarrhoea shedding.
From Table 4, the groups infected and treated with 40 mL of Ficus exasperata (C1 to C3) showed ruffled feathers and mild diarrhea of clinical signs within the pre-patent period of 3 to 7 days, while case mortality ranges from 1 to 2. For the groups treated with 20 mL extract of Ficus exasperata (D1 to D3), anoxoria and mucoid diarrhea were observed. Group E (positive control) had normal body activities and no case mortality was recorded in the group but group F (negative control) had bloody droppings and the case mortality increased to 3, which is also the group where case mortality was highest. It can also be seen from the Table 4 that the extract is effective for the treatment of coccidiosis in broiler chickens.
The ceacum of broiler chicken infected and treated with Ficus exasperata extract showed little tissue damage and absence of oocyst infection as shown in Plate 1. This signifies the effectiveness of the extracts on the broiler chickens and the economic advantage for poultry farmers.
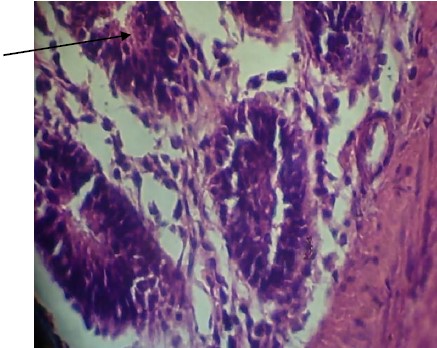
|
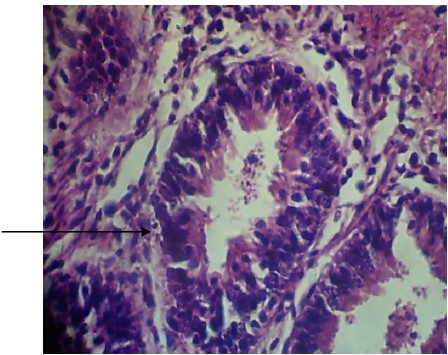
|
Plate 2 shows the ceacum of broiler chicken infected and treated with standard coccidial drug (positive control) showing less tissue damage and devoid of Eimeria oocyst. Comparing the results of histopathology obtained from the two plates one can easily confirm the effectiveness of the plant extracts since it has almost the same histopathology features as the standard coccidial drugs.
DISCUSSION
The results of this research work showed that within the pre-patent period of 3-5 days, the infection was established among birds in each of the groups except group E (positive control) which showed normal body activities. However, it was observed during this research work that broiler chicks that received dietary supplements of Ficus exasperata extracts for 2 weeks had a significant increase in body weight compared with the other groups2. Also, there was body weight gain in the group treated with standard anticoccidial drugs (embazine forte) but there was a drastic reduction in body weight of the group infected and left untreated (negative control)3. After the incidence of the disease, symptoms manifested and the signs that were noticed included reduced activities of the birds, ruffled feathers, reduced feed and water intake and mild diarrhea as shown in Table 4. The variation in body weight gain was experienced on week 7, one week post-challenge), this study also agreed with Belli et al.4 who stated that coccidial challenge had a significant effect on feed intake and body weight gain in broiler chicks. The histopathological examination showed that all the coccidian challenge birds carried developing oocysts in duodenum and caecum but the epithelial damage in general was not severe as this was supported by gross lesions and the low mortality rate5. Ficus exasperata extracts used for this research work numerically reduced the oocysts shedding within ten days post coccidial challenge when compared with the infected non-treated group, however at 14 days post-challenge a significant reduction of total oocysts excretion was observed between both challenged groups16. The use of different levels of plant extracts has shown to have potential benefits in reducing coccidian infection in broiler chickens infected with Eimeria tenella22.
The study implies that if the concentration of the extracts used for the treatment is too low, there will not be effective results on the broiler chickens. Over-concentration of the extracts also causes reactions among the birds. However, adequate prescribed concentration of the plant extracts gives optimum results. The limitation is that the plants are not readily available for use. It is therefore recommended that the plant extracts at appropriate concentration should be adequately used for the treatment of coccidiosis in poultry as it will serve as an alternative treatment to resistant drugs.
CONCLUSION
Ficus exasperata plant extract has the potential and effectiveness for the treatment of coccidiosis infection in broiler chickens infected with Eimeria tenella. The leaf extracts of the plant have little side effect on the broiler chickens compared with the high anticoccidial effects which it has on broiler chickens. However, precautions should be taken especially when administered to birds for the treatment of coccidiosis. Since the plant can prevent serious damage to the ceacum and duodenum of the infected broiler chickens, it can therefore be concluded that Ficus exasperata extracts have the potential to prevent coccidiosis in broiler chickens.
SIGNIFICANCE STATEMENT
This research work has shown the potential and effectiveness of Ficus exasperata extracts in treating coccidiosis infection in broiler chickens infected with Eimeria tenella, especially in the poultry industries. Due to the resistance of protozoan parasites present in infected broiler chickens and poultry in general, which render some of the standard coccidial drugs used in the poultry industry ineffective, there is a need to develop alternative therapy for the treatment of coccidiosis in broiler chickens and in the poultry industry. This research work has provided information on the future potential of the plant extract as good candidate for the treatment of chicken coccidiosis.
REFERENCES
- Allen, P.C. and R.H. Fetterer, 2002. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev., 15: 58-65.
- Bafundo, K.W., H.M. Cervantes and G.F. Mathis, 2008. Sensitivity of Eimeria field isolates in the United States: Responses of nicarbazin-containing anticoccidials. Poult. Sci., 87: 1760-1767.
- Bhanushali, J.K. and P.L. Long, 1985. Eimeria tenella infection: Does it affect humoral immune responses to heterologous antigens? J. Parasitol., 71: 850-852.
- Belli, S.I., N.C. Smith and D.J.P. Ferguson, 2006. The coccidian oocyst: A tough nut to crack! Trends Parasitol., 22: 416-423.
- Dalloul, R.A. and H.S. Lillehoj, 2005. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis., 49: 1-8.
- Fayer, R., 1980. Epidemiology of protozoan infections: The coccidia. Vet. Parasitol., 6: 75-103.
- Gharekhani, J., Z. Sadeghi-Dehkordi and M. Bahrami, 2014. Prevalence of coccidiosis in broiler chicken farms in Western Iran. J. Vet. Med., 2014.
- Giannenas, I., E. Tsalie, E. Triantafillou, S. Hessenberger, K. Teichmann, M. Mohnl and D. Tontis, 2014. Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol., 43: 209-216.
- Gibson, G.R. and M.B. Roberfroid, 1995. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr., 125: 1401-1412.
- Godwin, R.M. and J.A.T. Morgan, 2015. A molecular survey of Eimeria in chickens across Australia. Vet. Parasitol., 214: 16-21.
- Györke, A., L. Pop and V. Cozma, 2013. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite, 20.
- Hammond, D.M. and P.L. Long, 1973. The Coccidia: Eimeria, Isospora, Toxoplasma, and Related Genera. University Park Press, United States, ISBN: 9780839107323, Pages: 482.
- Innes, E.A. and A.N. Vermeulen, 2006. Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology, 133: S145-S168.
- Jadhav, B.N., S.V. Nikam, S.N. Bhamre and E.L. Jaid, 2011. Study of Eimeria necatrix in broiler chicken from Aurangabad District of Maharashtra State India. Int. Multidiscip. Res. J., 1: 11-12.
- Lee, J.T., C. Broussard, S. Fitz-Coy, P. Burke and N.H. Eckert et al., 2009. Evaluation of live oocyst vaccination or salinomycin for control of field-strain Eimeria challenge in broilers on two different feeding programs. J. Appl. Poult. Res., 18: 458-464.
- Lightowlers, M.W., 1994. Vaccination against animal parasites. Vet. Parasitol., 54: 177-204.
- McDonald, V. and M.W. Shirley, 2009. Past and future: Vaccination against Eimeria. Parasitology, 136: 1477-1489.
- Reid, M.W., 1990. History of avian medicine in the United States. X. Control of coccidiosis. Avian Dis., 34: 509-525.
- Ritzi, M.M., W. Abdelrahman, M. Mohnl and R.A. Dalloul, 2014. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge Poult. Sci., 93: 2772-2778.
- Ruff, M.D., 1999. Important parasites in poultry production systems. Vet. Parasitol., 84: 337-347.
- Shirley, M.W., A.L. Smith and F.M. Tomley, 2005. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv. Parasitol., 60: 285-330.
- Vermeulen, A.N., D.C. Schaap and T.P.M. Schetters, 2001. Control of coccidiosis in chickens by vaccination. Vet. Parasitol., 100: 13-20.
How to Cite this paper?
APA-7 Style
Henry,
K.I. (2023). The Use of Ficus exasperata Extracts for the Treatment of Coccidiosis in Poultry. Asian Journal of Biological Sciences, 16(4), 572-579. https://doi.org/10.3923/ajbs.2023.572.579
ACS Style
Henry,
K.I. The Use of Ficus exasperata Extracts for the Treatment of Coccidiosis in Poultry. Asian J. Biol. Sci 2023, 16, 572-579. https://doi.org/10.3923/ajbs.2023.572.579
AMA Style
Henry
KI. The Use of Ficus exasperata Extracts for the Treatment of Coccidiosis in Poultry. Asian Journal of Biological Sciences. 2023; 16(4): 572-579. https://doi.org/10.3923/ajbs.2023.572.579
Chicago/Turabian Style
Henry, Kolawole, Ilesanmi.
2023. "The Use of Ficus exasperata Extracts for the Treatment of Coccidiosis in Poultry" Asian Journal of Biological Sciences 16, no. 4: 572-579. https://doi.org/10.3923/ajbs.2023.572.579

This work is licensed under a Creative Commons Attribution 4.0 International License.


