Preventive and Ameliorative Effects of Diet Supplemented with Cucurbita maxima Leaf on Hyperglycemia and Hepatotoxicity in STZ-Induced Diabetic Rats
| Received 12 Sep, 2023 |
Accepted 18 Nov, 2023 |
Published 31 Dec, 2023 |
Background and Objective: Plant materials rich in antioxidant phytochemicals are able to offset the harmful effects of chemicals in the body. The antidiabetic and anti-hepatotoxic effects were investigated in STZ-induced diabetic rats fed a diet supplemented with Cucurbita maxima leaves. Materials and Methods: Sixty rats were randomly assigned into 6 groups of five animals each (n = 5) in preventive and ameliorative trials. Rats were supplemented with 5, 10, 15 and 20% Cucurbita maxima leaf for four weeks. The ANOVA in SPSS version 20 was used to examine the data followed by the Bonferroni multiple comparison (post hoc) Test. Results: Preliminary analysis of Cucurbita maxima leaf powder revealed antioxidant potential, with an IC50 for DPPH scavenging activity of 3.1 μg mg–1. The glucose levels and liver function parameters of the groups whose diets were supplemented with Cucurbita maxima leaf did not show signification (p>0.05) variations as compared to the normal control group in both preventive and ameliorative treatments. Conclusion: In both preventive and ameliorative trials, it can be concluded that the Cucurbita maxima leaves exhibit antihyperglycemic and anti-hepatotoxic effects.
| Copyright © 2023 Onuche et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
prevalence of diabetes mellitus (DM) worldwide continues to increase, making it a major challenge for global healthcare services1. Diabetes mellitus is connected to several critical issues that affect most bodily systems due to hyperglycemia and glucose intolerance, including hepatotoxicity, neuropathy, cardiovascular illnesses and renal disorders2. Insufficient intracellular glucose during DM harms and necrotizes hepatic cells, which promotes the apoptotic pathway. Pro-inflammatory responses have been observed in hepatic cells during DM3. Reactive oxygen species (ROS) generation and oxidative stress are two major factors in the pathogenesis of diabetic hepatotoxic damage4. Lipid peroxidation and oxidative necrosis occur when the intracellular production of free radicals exceeds the hepatocytes’ antioxidant system’s capacity. Due to hepatocyte injury, neutrophil infiltration and Kupffer cell activation produce inflammatory cytokines and apoptosis5.
Hyperglycemia is common in diabetic patients following trauma or a severe illness. According to Mahmoodpoor et al.6, most critically ill patients have insulin resistance, so blood sugar levels are difficult to control. In particular, the liver and skeletal muscles have a role in glucose metabolism, as do stress mediators such as stress hormones, cytokines and the central nervous system7. Systemic catecholamine release, cytokine release following systemic inflammation and direct systemic stimulation can all promote hepatic glycogenolysis and, eventually, hyperglycemia8.
Several medicinal plants with active compounds such as flavonoids, tannins, phenolics and alkaloids have hypoglycemic effects that help manage diabetes9. They also include antioxidant minerals like vitamin C, lutein and lycopene and, in some cases, substantial levels of crude fibre10. For instance, tannin enhances pancreatic beta-cell function and boosts insulin release. The antioxidant quercetin prevents lipid peroxidation and metal ion chelation through several mechanisms associated with eliminating oxygen radicals11. According to Hegazy et al.12, hypoglycemic plants raise insulin secretion, increase glucose absorption by muscle and fat tissues, limit glucose uptake from the intestine and inhibit glucose production by liver cells.
Due to its hypoglycemic activity, Cucurbita maxima has been used extensively in China and Mexico for diabetic patients8. Additionally, this herb is traditionally used for managing diabetes in Northwest Iran13. The C. maxima belongs to the family Cucurbitaceae and genus Cucurbita. The squash of C. maxima resembles a squash of Cucurbita mixta, Cucurbita pepo and Cucurbita moschota14. According to Mohaammed et al.14 and Onuche and Abu15, C. maxima is a giant pumpkin (English), Echi in Igala, Kabewa in Hausa, Anya in Igbo and Isi in Yoruba. It is a traditional crop cultivated in Nigeria for food and traditional medicine sources16 . Its leaves, fruits and seeds were boiled, roasted or baked. The leaf methanol extract possesses antioxidant activity and amylase inhibition17. Its leaf also has anti-anemic properties that could affect rats’ PVC, Hb and RBC counts16. A study by Onuche and Abu15 showed that the leaf of Cucurbita maxima affected colon carcinogenesis by significantly improving malondialdehyde (MDA) values of induced colon carcinogenesis in male albino rats. This study was conducted to determine the protective and ameliorative effects of C. maxima leaf supplementation in the standard feed of diabetic albino rats.
MATERIALS AND METHODS
Study area: The Cucurbita maxima leaf was bought from Ejule in Ofu LGA of Kogi State, Nigeria. The animal breeding, intoxication, treatments and biochemical analysis took place at the Biochemistry Laboratory of the Federal University of Agriculture Makurdi Benue State, Nigeria, whereas the data analysis and manuscript preparation were carried out at Federal University Wukari Taraba State, Nigeria. The study took six months to complete (March-August, 2023).
Plant collection and identification: Fresh Cucurbita maxima leaves (1 kg) were procured from Ejule in Ofu LGA of Kogi State and transported to a standard herbarium for identification, authentication and voucher issuance.
Plant preparation: Fresh Cucurbita maxima leaves were rinsed in clean water, dried at room temperature, ground into powder and then stored in an airtight container for further analysis.
Proximate analysis: Proximate analysis was carried out to determine moisture, crude fibre, crude lipid, crude protein and total ash by the AOAC methods18.
Determination of total phenol: The test tubes were filled with 1 mL of aliquots of the sample and gallic acid as a standard (10, 20, 40, 60, 80 and 100 g mL–1) and then 5 mL of distilled water and 0.5 mL of Folin Ciocalteu’s reagent were added and agitated. After 5 min of incubation, 10 mL of distilled water and 1.5 mL of 20% sodium carbonate were added, followed by re-incubation for 2 hrs at room temperature. After incubation, the absorbance of the sample was assessed using UV-visible Jasco V-630 Spectrophotometer, JASCO International CO. Ltd., 4-21, Sennin-cho 2-chome, Hachioji, Tokyo 193-0835, Japan equipment at 750 nm. The analysis was performed in triplicates. A reagent blank with solvent was used as a blank sample. Gallic acid was used to plot the calibration curve. The total phenolic content of Cucurbita maxima leaves was estimated as mg of gallic acid equivalent (GAE)/100 g of dry mass19.
Determination of tannins: Mujeeb et al.20 modified version approach was used to estimate tannins quantitatively. Cucurbita maxima leaves that had been finely pulverized were placed in a beaker with 20 mL of 50% methanol, covered with parafilm and heated for one hour in a water bath at 80°C with constant stirring. The extract was filtered using double-layered Whatman No. 1 filter paper with 50% methanol. One milliliter of extract was mixed with 20 mL of distilled water, 2.5 mL of Folin-Denis reagent and 10 mL of 17% Na2CO3 and left to stand for 20 min to produce a bluish-green color. The tannin content was determined by comparing the measured absorbance by UV-visible Jasco V-630 Spectrophotometer (JASCO International CO. Ltd., 4-21, Sennin-cho 2-chome, Hachioji, Tokyo 193-0835, Japan) at 760 nm to a reference curve established for a range of 0-10 ppm.
Determination of saponins: The Mujeeb et al.20 method determined the saponin content. One gram of finely powdered leaves was added with 100 mL of isobutyl alcohol and agitated for 5 hrs. The mixture was mixed with 20 mL of a 40% saturated magnesium carbonate solution and then filtered. One milliliter of solution was mixed with 2 mL of a 5% FeCl3 solution and 50 mL of distilled water and the mixture was left to stand for 30 min to develop the blood-red colour. The absorbance was measured at 380 nm using a UV-visible Jasco V-630 Spectrophotometer. A standard saponin curve was established for a 0-10 ppm range.
Determination of total flavonoid: Test tubes were filled with 1 mL of aliquots of the sample, 1 mL of standard quercetin solution (100, 200, 400, 600, 800 and 1000 g mL–1), 4 mL of distilled water and 0.3 mL of 5% sodium nitrite solution. After 5 min of incubation, 0.3 mL of 10% aluminum chloride was added and then 2 mL of 1 M sodium hydroxide was added at the 6th min. The volume was finally made up to 10 mL with distilled water and thoroughly mixed to produce an orange-yellow tint. The absorbance was measured using a UV-visible Jasco V-630 Spectrophotometer. Distilled water was used as a blank. Analysis was performed in duplicate. Standard quercetin was used to establish the calibration curve. The total flavonoid in the leaf powder was expressed as quercetin equivalent/g dry mass21.
Determination of antioxidant activity by DPPH: Two milliliters of a 1.0 mM DPPH radical solution in methanol were combined with one millilitre of a standard or extract solution ranging in concentration from 10 to 500 μg mL–1. After a quick stir, the solution was left to incubate in the dark at 37°C for 20 min. The UV-visible light was used to monitor the decline in absorbance of each solution. The spectrophotometer reading was taken at 517 nm on a Jasco V-630 from JASCO International Co. Ltd., at 4-21 Sennin-cho 2-chome, Hachioji, Tokyo 193-0835, Japan. The positive control was ascorbic acid, while the blank was a mixture of 1 millilitre of ethanol and 2 mL of a 1.0 mM DPPH radical solution22.
The degree of radical scavenging was measured by:
Where:
| Ac | = | Control absorbance | |
| As | = | Sample absorption |
By plotting the percentage of inhibition against the various concentrations, the concentration of sample needed to scavenge 50% of the free radical DPPH (IC50) was estimated.
Experimental diet: The experimental diet used in this study was grower mash feed produced by UAC Company from Jos, Plateau State. The feed served as the standard diet and was also used to mix with the powdered leaves of Cucurbita maxima to formulate dietary inclusion of 5, 10, 15 and 20% w/w) for the experimental group15.
Grouping of experimental animals: Sixty healthy male albino rats of the Wistar strain weighing 80-100 g were obtained from the National Veterinary Research Institute (NVRI), Vom, Plateau State, Nigeria. The experimental animals were kept in conventional laboratory settings (24 2°C, 12/12 hrs light-dark cycle), fed a standard grower mash pellet and given unlimited access to water for 28 days. The acclimatization period was carried out for two weeks before the experiment. All animals were weighed after the acclimatization period and assigned into 6 groups of 5 animals each for prevention and ameliorative treatments.
Induction of diabetes and treatments: The procedure of diabetic induction follows the method of Gurumallu et al.23. There are six treatment groups, each consisting of five animals. A single intraperitoneal injection of freshly prepared streptozotocin (STZ, 45 mg kg–1 b.wt.) in 0.1 M citrate buffer of pH 4.5 induced diabetes after an overnight fast.
Streptozotocin is a glucosamine derivative of nitrosourea that selectively destroys pancreatic islets of β-cells resulting in hyperglycemia and glycosuria. The STZ-induced rats with early-stage diabetes were treated with a 5% (w/v) glucose solution overnight to prevent death from hypoglycaemia. Rats’ blood sugar levels were tested 48 hrs later by pricking their tails and using a glucometer (Glucocard-01 Mini, Bengaluru).
Diabetic rats were those with fasting blood glucose levels >250 mg dL–1 that were included in the study. At the time of induction, the control group of rats received a single injection of 0.2 mL of vehicle (0.1 M citrate buffer at pH 4.5). Animals treated with STZ were given unlimited access to food and drink.
| Group 1: Normal control group received an equal volume of vehicle orally (P.O.)+normal (unformulated) feed | |
| Group 2: Induced diabetic group received normal feed (unformulated feed without supplementation of Cucurbita maxima leaf | |
| Group 3: Induced diabetic rats received 5% (w/w) Cucurbita maxima leaf formulated diet and water ad libitum | |
| Group 4: Diabetic rats received 10% (w/w) Cucurbita maxima leaf formulated diet and water ad libitum | |
| Group 5: Diabetic rats received 15% (w/w) Cucurbita maxima leaf formulated diet and water ad libitum | |
| Group 6: Diabetic rats received 20% (w/w) Cucurbita maxima leaf formulated diet and water ad libitum |
During the experimental period, the rats’ blood glucose levels and body weights were measured weekly, i.e., on days 0, 7, 14, 21 and 28. Biochemical parameters were analyzed on the 28th day. The rats were anesthetized with ether and the blood samples were collected and preserved for further analysis.
In the preventive study, STZ-induction was carried out after the experimental animals received a Cucurbita maxima leaf-formulated diet for four weeks. Consequently, the rats were sacrificed and the blood samples were collected and preserved for further analyses.
Determination of liver function: Liver function was assessed by analyzing serum concentrations of Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), total protein and albumin and direct and indirect bilirubin using an auto-chemistry analyzer (Cobas C111, Germany, Land wind LW E60B, China).
Ethical approval, statement of human and animal rights and statement of informed consent: All ethical protocols concerning the use of animals in scientific investigations of University of Agriculture Makurdi, Benue State, Nigeria were duly followed when conducting this experiment. All necessary permissions were taken through formal request and were duly granted before the conduct of the study.
Statistical analysis: The ANOVA in SPSS version 20 was used to examine the data (version 20 SPSS Inc., Chicago, Illinois, USA). The Bonferroni multiple comparison (post hoc) Test was used to compare differences among the various animal groups. Data was represented as Standard Deviation±Mean. The data with a p-<0.05 was significantly different.
RESULTS
Proximate composition of Cucurbita maxima leaves: The proximate composition of Cucurbita maxima leaves, including moisture, ash, moisture, ash, lipid, fibre, protein and carbohydrate, were 9.85±0.12, 5.43±0.05, 2.54±0.01, 8.56±0.03, 3.165±0.04 and 70.455±1.78 g/100 g dry matter, respectively as presented in Table 1. The result showed that the highest content of C. maxima leaf was carbohydrate.
Quantitative analysis of phytochemical content in Cucurbita maxima leaves: The quantitative analysis was carried out on total phenol, flavonoids, tannins and saponins. Total phenol was evaluated using the Folinciocalteu reagent method against a gallic acid standard curve using the linear regression equation
R2 = 0.8976
Total flavonoid was calculated using the aluminum chloride technique and the quercetin standard curve:
R2 = 0.864
The total phenol, flavonoids, tannins and saponins contents are presented in Table 2. Curcuma maxima leaves higher phenol concentration (6.32±0.41 mg GAE g–1 of extract), followed by flavonoid content with 3.21±0.09 mg QE g–1 extract.
| Table 1: | Proximate compositions of Cucurbita maxima Leaves | |||
| Parameter | Composition (dry matter (%)) |
| Moisture | 9.85±0.12 |
| Ash | 5.43±0.05 |
| Lipid | 2.54±0.01 |
| Fibre | 8.56±0.03 |
| Protein | 3.165±0.04 |
| Carbohydrate | 70.455±1.78 |
| n = 3 and Results are in Mean±Standard Deviation | |
| Table 2: | Quantitative phytochemical constituents of Cucurbita maxima leaves | |||
| Parameter | Amount (mg g–1) |
| Phenol | 6.32±0.41 |
| Tannins | 0.91±0.01 |
| Saponins | 1.26±0.01 |
| Flavonoids | 3.21±0.09 |
| n = 3 and Results are in Mean±Standard Deviation | |
|
|
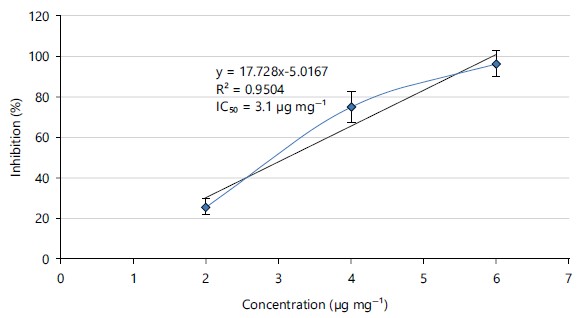
Antioxidant activity of Cucurbita maxima leaves: The ability of Cucurbita maxima leaves to scavenge DPPH free radicals was estimated by determining its IC50 using the linear regression equation in Fig. 1:
The free radical scavenging activity of Cucurbita maxima leaves increased as the extract doses increased. The IC50 of C. maxima was 3.1 μg mg–1.
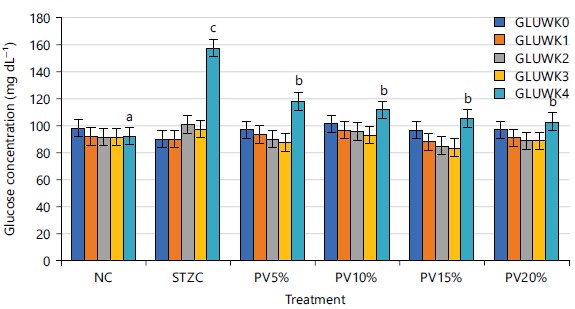
Preventive and ameliorative effects of feed supplemented with Cucurbita maxima leaves on blood glucose level in STZ-induced diabetic rats: Figure 2 depicts the weekly mean blood glucose of all rat groups in the preventative therapies from week 0 to week 4. All groups’ weekly glucose levels in weeks 0, 1, 2 and 3 were not significantly different (p>0.05). However, the STZ control group had a significantly (p<0.05) higher mean weekly blood glucose value in week 4 than the groups that received a C. maxima leaves-supplemented diet.

|
| Table 3: | Preventive effects of Cucurbita maxima leaves supplemented feed on liver function of STZ-induced diabetic rats | |||
| Treatment | ALT (IU L–1) |
AST (IU L–1) |
ALP (IU L–1) |
TB (mg dL–1) |
DB (mg dL–1) |
TP (mg dL–1) |
| NC | 43.00±8.27a |
58.47±7.95a |
110.45±3.43a |
1.50±0.54a |
0.08±0.00a |
59.83±5.43a |
| STZC | 109.14±5.91c |
114.85±2.25c |
226.17±5.14c |
5.88±0.06c |
4.18±0.01c |
33.50±1.63c |
| PV5% | 81.47±10.71b |
74.56±3.02b |
160.42±27.27b |
3.81±0.46b |
2.36±0.68b |
43.50±0.01b |
| PV10% | 67.54±9.06b |
60.32±1.53a |
175.36±1.03b |
2.45±0.56a |
0.50±0.01a |
40.75±0.54b |
| PV15% | 62.24±6.74a |
69.79±0.60a |
155.12±5.11b |
2.17±0.03a |
0.14±0.02a |
43.25±0.06b |
| PV20% | 59.41±7.86a |
48.69±5.52a |
142.77±9.92b |
2.49±0.11b |
0.07±0.00a |
42.76±0.45b |
| n = 5, Results are in Mean±Standard Deviation, values with different superscripts down the column are significantly different at (p<0.05), NC: Normal control, STZC: Streptozotocin, PV: Preventive treatment, ALT: Alanine transaminase, AST: Aspartate transaminase, ALP: Alkaline phosphatase, TB: Total bilirubin, DB: Direct bilirubin and TP: Total protein | ||||||
| Table 4: | Ameliorative effects of Cucurbita maxima leaves supplemented feed on liver function of STZ-induced diabetic rats | |||
| Treatment | ALT (IU L–1) |
AST (IU L–1) |
ALP (IU L–1) |
TB (mg dL–1) |
DB (mg dL–1) |
TP (mg dL–1) |
| NC | 43.00±8.27a |
58.47±7.95a |
110.45±3.43a |
1.50±0.54a |
0.08±0.00a |
59.83±5.43b |
| STZC | 109.14±5.91b |
114.85±2.25c |
226.17±5.14b |
5.88±0.06c |
4.18±0.01b |
33.50±1.63c |
| AM5% | 47.08±5.49a |
81.39±10.75b |
115.06±7.53a |
2.94±0.55b |
0.04±0.01a |
40.15±0.05a |
| AM10% | 41.39±17.69a |
68.47±7.14a |
108.61±8.48a |
2.53±0.50b |
0.15±0.00a |
43.53±0.14a |
| AM15% | 53.20±5.80a |
55.83±11.05a |
102.86±3.11a |
2.44±0.52a |
0.05±0.00a |
45.62±0.54a |
| AM20% | 58.00±12.57a |
57.16±10.59a |
109.11±10.57a |
2.12±0.44a |
0.17±0.00a |
43.01±0.57a |
| n = 5, Results are in Mean±Standard Deviation, values with different superscripts down the column are significantly different at (p<0.05), NC: Normal control, STZC: Streptozotocincontrol, AM: Ameliorative treatment, ALT: Alanine transaminase, AST: Aspartate transaminase, ALP = Alkaline phosphatase, TB: Total bilirubin, DB: Direct bilirubin and TP: Total protein | ||||||
Figure 3 represents the mean blood glucose in the ameliorative treatments from week 0 to 4. The weekly mean glucose levels of the groups did not differ significantly (p>0.05) in week 0. However, the glucose level in the STZ control group increased significantly (p<0.05) from week 2 to 4, whereas the groups that received a C. maxima leaves-supplemented diet reduced significantly (p<0.05) from week 2 to 4.
Preventive and ameliorative effects of Cucurbita maxima leaves supplemented diet on liver function parameters of STZ-induced diabetic rats: Table 3 reveals that STZ induction did not cause significant (p>0.05) changes in the liver function parameters in the supplemented groups of preventive and ameliorative treatments except for the STZ control group when compared to the normal control group after 28 days of C. maxima leaves supplementation.
Table 4 shows a substantial (p<0.05) reduction in ALT, AST, ALP, TB and DB levels in the rats that received a C. maxima leaves-supplemented diet compared to the diabetic non-supplemented control group (STZ control). Total protein, on the other hand, increased significantly (p<0.05) in the supplemented groups compared to the diabetic non-supplemented control group.
DISCUSSION
Supplemented diets of Cucurbita maxima provided efficient protective and ameliorative properties against streptozotocin-induced diabetes in rat experimental models by restoring glucose metabolism anomalies and normalizing liver function parameters. All the experimental rats fed with diets supplemented with C. maxima demonstrated significant reversal of the biochemical parameters evaluated which was comparable with normal control. On the other hand, the negative control showed significant variations in all the biochemical indices measured when compared with the normal control. The study therefore provided possible agents for the management of diabetes and its associated risks such as distortion of the liver function.
The adverse effects of synthetic medications encourage the search for new medicinal sources for metabolic diseases. According to Okur et al.24, herbal medicine has emerged as a promising treatment for effectively managing diabetes. The present research revealed that the C. maxima leaf had high amounts of carbohydrates, moisture and fibre. It was consistent with the previous study by Jahan et al.25. Cucurbita maxima’s edible sections may aid a person in consuming the appropriate amount of micronutrients daily26.
Bioactive components of plants could be used to treat oxidative stress, chronic illnesses and aging. Nutraceuticals of plant origin, often known as phytochemical compounds, have been suggested by Cicero and Colletti27 as a potential treatment for metabolic syndrome. The phytochemicals and their effects on diabetes and associated consequences were also listed in the report. A preliminary phytochemical study was conducted to determine the presence of flavonoids, phenols, tannins and saponins in the powder of C. maxima leaves. Phenols and flavonoids exhibit antioxidant, antibacterial, anti-inflammatory, anti-diabetic and anti-cancer properties28.
The risk of metabolic syndrome and its associated complications, such as type 2 diabetes, may be reduced by consuming phenolic substances such phenolic acids and flavonoids. However, little is known about the processes through which the various classes of phenolic chemicals protect illness29.
By using the radical DPPH (2,2-diphenyl-1-picrylhydrazyl), which transforms into a stable diamagnetic molecule30, it is possible to measure the strength of free radicals that are created by DPPH31. Results on IC50 showed that, C. maxima leaf supplemented diet demonstrated antiradical activity. Increasing antiradical activity is indicated by the smaller IC50 value32.
According to Nasr et al.33, oxidative stress is caused by oxidant formation and relates to diabetic problems. In the current investigation, albino rats were given an intraperitoneal injection of STZ at a dosage of 45 mg kg–1 body weight. According to Sunil et al.23, streptozotocin (STZ) is frequently used to induce diabetes in animal models and a single STZ injection can cause diabetes by damaging Langerhans Islets in the pancreas. Streptozotocin specifically recognizes the abundantly present glucose transporter two receptors on plasma membranes of cells23.
The blood glucose levels of all diabetic rat groups receiving preventative and ameliorative therapies were considerably (p<0.05) higher than those of the negative control rat group. Streptozotocin administration results in insulin suppression, followed by increasing blood glucose excessively which agreed with the observation of Damasceno et al.34.
Results in this study showed that in the preventative and ameliorative treatments, the C. maxima leaf supplementation diet in diabetic rats lowered the blood glucose levels significantly (p<0.05) when compared to the diabetic control rat group that had not received the C. maxima leaf supplemented diet. The drop in blood glucose levels in STZ-induced diabetic rats provided with a supplemental diet containing C. maxima leaf shows the hypoglycemic effect of the feed additions. The C. maxima leaf supplementation may have an anti-diabetic impact because the phytochemical compounds might have an insulin-like effect or they cause the pancreatic islet β-cells to secrete more insulin after consuming the diet-supplemented C. maxima leaf35.
Persistent hyperglycemia and insulin insufficiency or resistance usually damage liver and renal tissue36. The standard liver function parameters for identifying liver disease in clinical practice are bilirubin, ALT, AST and ALP. Bilirubin is usually processed in the liver as a by-product of heme catabolism and expelled from the body. The liver’s reduced function causes an increase in bilirubin in the blood. Albumin gene transcription is decreased by insulin insufficiency, which lowers serum albumin levels37. According to Saleh et al.38, liver injury is indicated by high levels of bilirubin, ALT, AST and ALP and low levels of albumin and protein in the blood.
In the current investigation, hepatocyte injury was indicated by a substantial rise in liver function parameters (p<0.05) in the serum of the STZ-induced diabetic control rat group which collaborated with the report of Pulivarthi et al.39. According to Al-Bahrani40 and Saleh et al.38, high levels of these enzymes in the serum of STZ-induced diabetic rats represent hepatocyte damage. Streptozotocin specifically recognizes glucose transporter 2 receptors on the cell plasma membrane23 and these receptors are also present on the liver and kidney cell membranes41. Therefore, STZ induction in animal models may also interfere with hepato-renal function41.
When compared to the diabetic control rat group, the levels of bilirubin, ALT, AST and ALP in the serum of STZ-induced diabetic rats who received a diet supplemented with C. maxima leaves were reduced significantly (p<0.05). Compared to the diabetic control rat group following treatment with a supplemented diet, the serum levels of the liver enzymes trended downward to normal levels, which may be attributed to cell membrane stability and cellular regeneration brought about by the interaction of bioactive components in the plant material with the cellular receptors42. These findings show that plant-based supplements have hepatoprotective properties.
The results suggest that C. maxima inhibits STZ-induced hyperglycemia and hepatotoxicity in diabetic rats and that these effects may be mediated by interacting with multiple receptors to raise the levels of antioxidant enzymes in the system. However, the actual mechanism of biological activities of the plant’s active components was not elucidated in the present study and could be a limiting factor in harnessing the full potential of the plant.
CONCLUSION
The Cucurbita maxima leaf is abundant in phytochemicals. It has potential antihyperglycemic activity and can protect against and treat biochemical changes related to diabetes. The current study provided scientific support for the traditional use of C. maxima leaf as a blood glucose-lowering plant. Further study to evaluate the precise mechanism of the active constituents of C. maxima leaf, determine various bioactive compounds in the C. maxima leaf and isolate active compounds responsible for lowering blood glucose should be considered.
SIGNIFICANCE STATEMENT
Diabetes is yet to have a permanent cure and therefore often poses a severe burden to the patient and society in general. On this premise, Cucurbita maxima were investigated for antidiabetic properties to increase the horizon of the available treatment options for diabetes. Supplemented diets of Cucurbita maxima provided efficient protective and ameliorative properties against streptozotocin-induced diabetes in rat experimental models by restoring glucose metabolism anomalies and normalizing liver function parameters. Hence, the study has added possible agents in the management of diabetes and its associated risks such as distortion of the liver function.
REFERENCES
- Khan, M.A.B., M.J. Hashim, J.K. King, R.D. Govender, H. Mustafa and J. Al Kaabi, 2020. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends. J. Epidemiol. Global Health, 10: 107-111.
- Targher, G., 2019. Is it time for non-alcoholic fatty liver disease screening in patients with type 2 diabetes mellitus? Hepatobiliary Surg. Nutr., 9: 239-241.
- Lee, H. and Y. Lim, 2019. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr. Res. Pract., 13: 377-383.
- Abulikemu, A., X. Zhao, H. Xu, Y. Li and R. Ma et al., 2023. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol., 59.
- Zeng, H. and Z. Liu, 2019. Atorvastatin induces hepatotoxicity in diabetic rats via oxidative stress, inflammation, and anti-apoptotic pathway. Med. Sci. Monit., 25: 6165-6173.
- Mahmoodpoor, A., H. Hamishehkar, K. Shadvar, M. Beigmohammadi, A. Iranpour and S. Sanaie, 2016. Relationship between glycated hemoglobin, intensive care unit admission blood sugar and glucose control with ICU mortality in critically ill patients. Indian J. Crit. Care Med., 20: 67-71.
- Kawahito, S., H. Kitahata and S. Oshita, 2009. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol., 15: 4137-4142.
- Huerta-Reyes, M., R. Tavera-Hernández, J.J. Alvarado-Sansininea and M. Jiménez-Estrada, 2022. Selected species of the Cucurbitaceae family used in Mexico for the treatment of diabetes mellitus. Molecules, 27.
- Kooti, W., M. Farokhipour, Z. Asadzadeh, D. Ashtary-Larky and M. Asadi-Samani, 2016. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Phys., 8: 1832-1842.
- Neela, S. and S.W. Fanta, 2019. Review on nutritional composition of orange-fleshed sweet potato and its role in management of vitamin A deficiency. Food Sci. Nutr., 7: 1920-1945.
- Qi, W., W. Qi, D. Xiong and M. Long, 2022. Quercetin: Its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules, 27.
- Hegazy, G.A., A.M. Alnoury and H.G. Gad, 2013. The role of Acacia arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med. J., 34: 727-733.
- Mahmoodpoor, A., M. Medghalchi, H. Nazemiyeh, P. Asgharian, K. Shadvar and H. Hamishehkar, 2018. Effect of cucurbita maxima on control of blood glucose in diabetic critically Ill patients. Adv. Pharm. Bull., 8: 347-351.
- Mohaammed, S.S., Y.B. Paiko, A. Mann, M.M. Ndamitso, J.T. Mathew and S. Maaji, 2014. Proximate, mineral and anti-nutritional composition of Cucurbita maxima fruits parts. Niger. J. Chem. Res., 19: 37-49.
- Onuche, J.I. and M.S. Abu, 2021. Assessment of the preventive effect of dietary inclusion of Cucurbita maxima (Duch) leaf on N-methyl-N-nitrosourea (MNU) induced colon carcinogenesis in Wistar rats. Appl. Biol. Chem. J., 2: 93-101.
- Yongabi, K.A., E.F. Fon, H. Lukong and P.N. Chia, 2014. A preliminary assessment of Cucurbita maxima leaves from cameroon on haematological parameters in albino rats. J. Mol. Pharm. Org. Process Res., 2.
- Al-Shaheen, S.J.A., R.A. Kaskoos, K.J. Hamad and J. Ahamad, 2013. In-vitro antioxidant and α-amylase inhibition activity of Cucurbita maxima. J. Pharmacogn. Phytochem., 2: 121-124.
- AOAC., 2000. Official Methods of Analysis of AOAC International. 17th Edn., Association of Official Analytical Chemists, Gaithersburg, Maryland.
- Bhalodia, N., P. Nariya, V. Shukla and R. Acharya, 2013. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula linn. Int. Q. J. Res. Ayurveda, 34: 209-214.
- Mujeeb, F., P. Bajpai and N. Pathak, 2014. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int., 2014.
- Kalita, P., B.K. Tapan, T.K. Pal and R. Kalita, 2013. Estimation of total flavonoids content (tfc) and anti oxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Delivery Ther., 3: 33-37.
- Baliyan, S., R. Mukherjee, A. Priyadarshini, A. Vibhuti, A. Gupta, R.P. Pandey and C.M. Chang, 2022. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules, 27.
- Gurumallu, S.C., T.N. AlRamadneh, H.N. Sarjan, A. Bhaskar, C.M.F. Pereira and R. Javaraiah, 2022. Synergistic hypoglycemic and hypolipidemic effects of ω-3 and ω-6 fatty acids from Indian flax and sesame seed oils in streptozotocin-induced diabetic rats. Phytomed. Plus, 2.
- Okur, M.E., I.D. Karantas and P.I. Siafaka, 2017. Diabetes mellitus: A review on pathophysiology, current status of oral medications and future perspectives. Acta Pharm. Sci., 55: 61-82.
- Jahan, F., M. Badrul Islam, S.P. Moulick, M. Al Bashera and M.S. Hasan et al., 2023. Nutritional characterization and antioxidant properties of various edible portions of Cucurbita maxima: A potential source of nutraceuticals. Heliyon, 9.
- Kostecka-Gugała, A., M. Kruczek, I. Ledwożyw-Smoleń and P. Kaszycki, 2020. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules, 25.
- Cicero, A.F.G. and A. Colletti, 2016. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine, 23: 1134-1144.
- Tungmunnithum, D., A. Thongboonyou, A. Pholboon and A. Yangsabai, 2018. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicine, 5.
- Lin, D., M. Xiao, J. Zhao, Z. Li and B. Xing et al., 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules, 21.
- Mishra, K., H. Ojha and N.K. Chaudhury, 2012. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem., 130: 1036-1043.
- Rohman, A., S. Riyanto, Mistriyani, Shuhaira and A.E. Nugroho, 2017. Antiradical activities of rambutan peel: Study from two cultivars. Res. J. Phytochem., 11: 42-47.
- Bērziņa, L. and I. Mieriņa, 2023. Antiradical and antioxidant activity of compounds containing 1,3-dicarbonyl moiety: An overview. Molecules, 28.
- Nasr, H.M., F.M. El-Demerdash and W.A. El-Nagar, 2016. Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats: Toxicity of insecticide mixture. Environ. Sci. Pollut. Res., 23: 1852-1859.
- Damasceno, D.C., A.O. Netto, I.L. Lessi, F.Q. Gallego and S.B. Corvino et al., 2014. Streptozotocin-induced diabetes models: Pathophysiological mechanisms and fetal outcomes. BioMed Res. Int., 2014.
- Pirmoghani, A., I. Salehi, S. Moradkhani, S.A. Karimi and S. Salehi, 2019. Effect of crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep., 7: 90-96.
- Jaiswal, Y.S., P.A. Tatke, S.Y. Gabhe and A.B. Vaidya, 2017. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J. Tradit. Complementary Med., 7: 421-427.
- Bae, J.C., S.H. Seo, K.Y. Hur, J.H. Kim and M.S. Lee et al., 2013. Association between serum albumin, insulin resistance, and incident diabetes in nondiabetic subjects. Endocrinol. Metab., 28: 26-32.
- Saleh, N.S., T.S. Allam, R.M. El-Rabeaie and H.S. El-Sabbagh, 2018. Protective effect of some Egyptian medicinal plants against oxidative stress in rats. Alexandria J. Vet. Sci., 59: 1-14.
- Pulivarthi, V., P. Josthna and C.V. Naidu, 2021. Ameliorative effect of Annona reticulata L. leaf extract on antihyperglycemic activity and its hepato-renal protective potential in streptozotocin induced diabetic rats. J. Ayurveda Integr. Med., 12: 415-426.
- Al-Bahrani, M.H.A., 2016. The role of Momordica charantia in reducing the level of glucose in mice. Int. J. Curr. Microbiol. Appl. Sci., 5: 470-478.
- Bouwens, L. and I. Rooman, 2005. Regulation of pancreatic beta-cell mass. Physiol. Rev., 85: 1255-1270.
- Madrigal-Santilln, E., E. Madrigal-Bujaidar, S.C. Jaime, M. del Carmen Valadez-Vega, M. Teresa, K. Guadalupe and J. Antonio, 2013. The Chemoprevention of Chronic Degenerative Disease Through Dietary Antioxidants: Progress, Promise and Evidences. In: Oxidative Stress and Chronic Degenerative Diseases-A Role for Antioxidants, Morales-Gonzalez, J.A. (Ed.), InTech, London, UK, ISBN: 978-953-51-1123-8.
How to Cite this paper?
APA-7 Style
Onuche,
J.I., Adebisi,
A.K., Ikwebe,
J., Abu,
M.S. (2023). Preventive and Ameliorative Effects of Diet Supplemented with Cucurbita maxima Leaf on Hyperglycemia and Hepatotoxicity in STZ-Induced Diabetic Rats. Asian Journal of Biological Sciences, 16(4), 502-513. https://doi.org/10.3923/ajbs.2023.502.513
ACS Style
Onuche,
J.I.; Adebisi,
A.K.; Ikwebe,
J.; Abu,
M.S. Preventive and Ameliorative Effects of Diet Supplemented with Cucurbita maxima Leaf on Hyperglycemia and Hepatotoxicity in STZ-Induced Diabetic Rats. Asian J. Biol. Sci 2023, 16, 502-513. https://doi.org/10.3923/ajbs.2023.502.513
AMA Style
Onuche
JI, Adebisi
AK, Ikwebe
J, Abu
MS. Preventive and Ameliorative Effects of Diet Supplemented with Cucurbita maxima Leaf on Hyperglycemia and Hepatotoxicity in STZ-Induced Diabetic Rats. Asian Journal of Biological Sciences. 2023; 16(4): 502-513. https://doi.org/10.3923/ajbs.2023.502.513
Chicago/Turabian Style
Onuche, Job, Itanyi, Arowora Kayode Adebisi, Joseph Ikwebe, and Michael Sunday Abu.
2023. "Preventive and Ameliorative Effects of Diet Supplemented with Cucurbita maxima Leaf on Hyperglycemia and Hepatotoxicity in STZ-Induced Diabetic Rats" Asian Journal of Biological Sciences 16, no. 4: 502-513. https://doi.org/10.3923/ajbs.2023.502.513

This work is licensed under a Creative Commons Attribution 4.0 International License.



