Post-Harvest Handling Survey Report on Cocoyam [Colocasia esculenta (L.) Schott] in Oji-River, Enugu State, Nigeria
| Received 17 Sep, 2023 |
Accepted 10 Nov, 2023 |
Published 31 Dec, 2023 |
Background and Objective: This study presents a survey on the post-harvest handling practices of cocoyam and the identification of fungi responsible for cocoyam rot. The objectives are to assess the perception of farmers on the pre and post-harvest handling practices of cocoyam and to identify the fungi responsible for cocoyam rot. Materials and Methods: The survey was conducted from November, 2022 to May, 2023 in Oji River Local Government Area, Enugu State. A total of 120 well-structured questionnaires on handling practices of cocoyam were administered to 20 randomly selected cocoyam farmers/traders in each of the 6 communities in Oji River. Isolation of pathogens from cocoyam cormels with symptoms of rot purchased from Oji River was done by inoculating a portion of the explant onto sabouraud dextrose agar. The isolates were sub-cultured and identified. Data were analyzed using descriptive statistics. Results: The survey revealed that the majority of cocoyam farmers/sellers were female (61.67%), within the age range of 41-15 (23.33%), the majority had post primary education (49.17) while 5% had no formal education. All the respondents (100%) had knowledge of fungi diseases and could identify various symptoms of fungi infections in plants. Chiefly among the methods of pre and post-harvest rot control in cocoyam were crop rotation (88.33%) and proper storage (95%). The organisms isolated were Aspergillus niger, Rhizopus stolonifer, Mucor circinelloides, Pennicillium expansum and Fusarium oxysporum. The pathogenicity test showed that the test organisms were responsible for cocoyam spoilage. Conclusion: Proper care should be taken in the handling of cocoyam as some of these spoilage fungi are known to have a negative impact.
| Copyright © 2023 Azubuike et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Cocoyams (Colocasia esculenta) are herbaceous perennial plants belonging to the family Araceae and are grown primarily for their edible roots and used as food in China and many African countries including Nigeria and Ghana. Cocoyam is one of the most important root crops in Nigeria1. Africa is the highest producer of cocoyam, it is on record that most countries in West Africa such as Nigeria, Ghana and Cameroon contribute over 60% of the total African production of cocoyam2. This postulates that the importance of cocoyam to regional food security cannot be overemphasized. Cocoyam has a high productivity level and better storability compared to other tropical root and tuber crops3. Cocoyam has been marginalized in agricultural policies and research interventions on root and tuber crops. It remains an underexploited food resource4. Cocoyam is best cultivated under direct sunlight, it can tolerate shade and in most cases intercropped with oil palms, bananas and other perennial crops. This is more common, especially at the early stage of these plantations. Cocoyam production is generally found among small-scale farmers where its production is undermined5. Hence, the production of cocoyam has not been given proper attention in many countries probably because of its incapability to earn foreign exchange and its unacceptability by high-end consumer’s income countries for both consumption and other purposes. Cocoyam is widely consumed by people because of its nutritional content and other values.
The reports of Gollifer and Booth6, show that cocoyam has a relatively short post-harvest storage life of about 7 days. Biodegradative losses are very high which may result in complete maceration of the tissues and 100% losses as a result of storage pathogens7.
Most post-harvest losses of cocoyam tubers in storage are associated with a number of physical and physiological factors arising from harvesting, storage or transportation. These cause spoilage or deterioration of the tubers8. Pathogen invasion through natural openings such as stomata or wounds is one of the most important factors in cocoyam spoilage9,10.
It has been suggested that November, March and April are the ideal time for harvesting cocoyam. However, due to the difficulties in storage, cocoyam is usually utilized or consumed fresh shortly after harvest because of these fungi which lead to their spoilage during storage11.
The study was aimed at surveying the perception of cocoyam farmers/traders on the post-harvest handling practices of cocoyam and the identification of fungi responsible for cocoyam rot in Oji River Local Government, Enugu State.
MATERIALS AND METHODS
Study area: This study was conducted in Oji River Local Government Area, Enugu State. The local government is made up of six towns namely Achi, Inyi, Awlaw, Akpugoeze, Ugwuoba and Oji Town. Oji River Local Government has an area of 403 km2 and a population of 126,587. It is located within the Latitude 6.1703°N and Longitude 7.2772°E. Oji River has one of the largest and oldest running leprosy rehabilitation settlements in the Southeast, bordering Anambra state. It had been originally set up by English Missionary Churches in the 1930s in Nigeria.
Survey: The survey was conducted between November, 2022 to May, 2023 in six towns in Oji River Local Government Area (Achi, Inyi, Awlaw, Akpugoeze, Ugwuoba and Oji town). A well-structured questionnaire was used as the instrument for data collection. The questionnaire was administered randomly to a total of 120 respondents, 20 respondents from each town. These respondents are cocoyam traders/farmers in various communities. The first section of the questionnaire was in the Socio-demographic details of the respondents while the second part included questions about the pre and post-harvest handling processes of cocoyam.
Collection of sample: Colocasia esculenta L. cormels showing symptoms of rot were randomly selected and purchased from six different towns in Oji River Local Government Area in Enugu State Nigeria. The towns are Achi, Inyi, Ugwuoba, Awlaw, Akpugoeze and Oji River urban. Seven cormels of cocoyam with symptoms of rot were collected from each town making a total of forty-two samples in all. The samples were collected in sterile polythene bags, well-labelled for easy identification and taken to the Department of Botany Laboratory, Nnamdi Azikiwe University, Awka for further analysis. The samples were identified and authenticated by Mr. Chisom Iloka, a Technologist in the Department of Botany.
Fungal isolation: This was carried out by the method of Chikwendu et al.12. Fungal isolation was done by serial dilution. The cocoyam sample was weighed (1 g), suspended in 9 mL sterile water in a test tube (10-1) and shaken well. An aliquot of 1 mL was transferred from this dilution into the second test tube containing 9.0 mL of sterilized water to arrive at 10-2. Another 1.0 mL was transferred from this second test tube (10-2) to the third test tube containing 9.0 mL of sterile distilled water 10-3. This was repeated until 10-6. At the end of the serial dilution, 0.1 mL of the solution was transferred from 10-5 and 10-6 dilutions of each sample. This was followed by inoculation on solidified plates of oil agar. The plates were incubated at 30°C for 7 days with daily observation, after which it was sub-cultured in a fresh sabouraud dextrose agar to get a pure culture using the streak method.
Characterization and identification of fungi: The fungi were characterized and identified based on their colonial and microscopic characteristics. Lactophenol cotton blue staining and slide culture tests were carried out to characterize the fungal species using the methods described by Raja et al.13. The fungi were identified following the morphological structures of the fungi such as hyphae (septation) and reproductive structures (sporangia/conidia) in chain or single, the type of spore was observed and recorded as described by Okigbo et al.14.
Pathogenicity test: The method of Ezugwu15 was used for the pathogenicity test. This was carried out using nine healthy cocoyam cormels. The cormels were washed with tap water, rinsed with distilled water and then surface sterilized with 75% ethanol. Measuring the pathogenicity of the rot-causing fungi was done by obtaining the rotted portions of the whole tubers and taking the final weight of the individual cocoyam cormel. Un-inoculated cormel serves as the control.
Four test fungi: Rhizopus stolonifer, Aspergillus niger, Fusarium oxysporum and Mucor circinelloides were used for the study. Healthy cocoyam cormels were washed and surface sterilized with 70% ethanol. The cormels were placed on sterile paper towels and allowed to dry for 12 min in a laminar airflow hood (4F ETL Cabinet 220V, California, USA). Holes were bored in the cocoyam cormels using a sterile cork borer.
An agar block was inoculated into the hole made with the aid of another cork borer, after the inoculation the parts of the cormel bore out were carefully replaced and sealed with sterile vaseline to prevent contamination and labeled accordingly. The control experiment was set up with a tuber that bore no isolate but was inoculated with 1 mL of sterile distilled water.
After inoculating the entire test isolates into their respective healthy cormels and incubated for 6 days in a humidity chamber. The cormels were checked daily for sign of rot. At the end of the incubation period, the cormels were carefully cut open along the line of inoculation to expose the regions rot on the inoculated cormels. The percentage severity of rot (Sr (%)) was calculated according to the method of Lamini et al.16:
Where:
| FW | = | Final weight of infected cocoyam cormel | |
| w | = | Weight of rotted cormel portion |
Statistical analysis: Statistical analysis was done using descriptive statistics for both the survey and the microbial analysis. Frequency count and percentage were used to describe the data collected from the survey.
RESULTS
Survey: The inference of the survey conducted on cocoyam in Oji River Local Government Area, Enugu State is presented below. The survey captured the socio-demographic details of the respondents and their perception of the diseases of cocoyam and the post-harvest rot control methodologies.
Socio-demographic characteristics of the respondents: The socio-demographic data collected from the survey showed that the majority of the respondents are within the age range 41-45 and 46-50 with frequencies of 28 (23.33%) and 25 (20.83%), respectively. The age range with the least number of respondents is 36-40 and 56-60 with frequencies of 15 (12.50%) each. It was also observed that the majority of the respondents were female (61.67%) while 38.33% were male. Most of the respondents had SSCE (49.17%) and FSLC (45.83%), while just 5% had no formal education (Table 1).
Knowledge and perception of cocoyam farmers/traders on the diseases of cocoyam: As the 120 respondents who were able to identify fungal disease, yellowing and leaf spots were the most identified while reduced leaf size and necrosis were the least. 5-9 years have higher experience while more than 24 years have the lowest (Table 2).
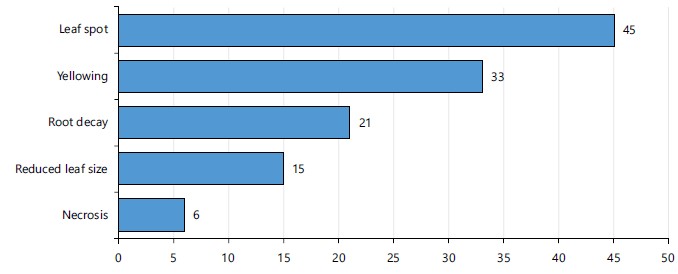
Knowledge of farmers on fungal damage to cocoyam: Determining the knowledge of farmers on the fungal damage of cocoyam shows that 45% of the respondents reported that fungi cause leaf spot, 33% agreed that fungi cause yellowing while only 6% said that fungi cause necrosis (Fig. 1).
| Table 1: | Socio-demographic characteristics of the study participants | |||
| Variables | Frequency (n = 120) |
Percentage |
| Selected communities | ||
| Achi | 20 |
16.67 |
| Akpugoeze | 20 |
16.67 |
| Awlaw | 20 |
16.67 |
| Inyi | 20 |
16.67 |
| Oji River urban | 20 |
16.67 |
| Ugwuoba | 20 |
16.67 |
| Age | ||
| 36-40 | 15 |
12.5 |
| 41-45 | 28 |
23.33 |
| 46-50 | 25 |
20.83 |
| 51-55 | 20 |
16.67 |
| 56-60 | 15 |
12.5 |
| Above 60 | 17 |
14.17 |
| Sex | ||
| Female | 74 |
61.67 |
| Male | 46 |
38.33 |
| Marital status | ||
| Divorced | 1 |
0.83 |
| Single | 3 |
2.5 |
| Married | 116 |
96.67 |
| Highest educational level | ||
| No formal education | 6 |
5 |
| FSLC | 55 |
45.83 |
| SSCE | 59 |
49.17 |
| Total | 120 |
100 |
| FLSC: First school leaving certificate and SSCE: Senior school certificate examination | ||
|
| Table 2: | Knowledge and perception of cocoyam farmers/traders on the diseases of cocoyam | |||
| Variable | Frequency (n=120) |
Percentage |
| Can you identify fungal diseases? | ||
| Yes | 120 |
100 |
| Damages done to cocoyam by fungi | ||
| Necrosis | 6 |
5 |
| Reduced leaf size | 15 |
12.5 |
| Root decay | 21 |
17.5 |
| Yellowing | 33 |
27.5 |
| Leaf spot | 45 |
37.5 |
| Experience in cocoyam cultivation | ||
| 5-9 years | 75 |
62.5 |
| 10-14 years | 42 |
35 |
| 15-19 years | 2 |
1.67 |
| More than 24 years | 1 |
0.83 |
| Total | 120 |
100 |
| Table 3: | Response from study participants on the pre and post-harvest strategies for controlling diseases of cocoyam in some selected communities in Oji River LGA | |||
| Variable | Frequency (n = 120) |
Percentage |
| Pre-harvest rot control methodologies | ||
| Pest control | 1 |
0.83 |
| Resistant varieties | 2 |
1.67 |
| Irrigation | 11 |
9.17 |
| Crop rotation | 106 |
88.33 |
| Post-harvest control strategies | ||
| Pesticides | 2 |
1.67 |
| Ash/ammoniation | 4 |
3.33 |
| Proper storage | 114 |
95 |
Cocoyam farmers/traders response on the pre and post-harvest strategies for controlling diseases of cocoyam: The majority of the respondents identified crop rotation as a means of pre-harvest rot control and only one person identified pest control. Most farmers use proper storage as a post-harvest control strategy while only 2 people use pesticides (Table 3).
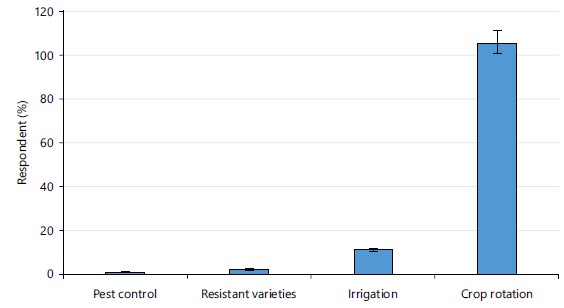
Pre-harvest handling strategies for controlling diseases of cocoyam: Only one respondent mentioned pest control as a way of controlling diseases of cocoyam while two respondents mentioned the use of resistant varieties. The majority of the respondents (106) agreed that crop rotation is the major means of controlling pre-harvest rot of cocoyam (Fig. 2).
|
|
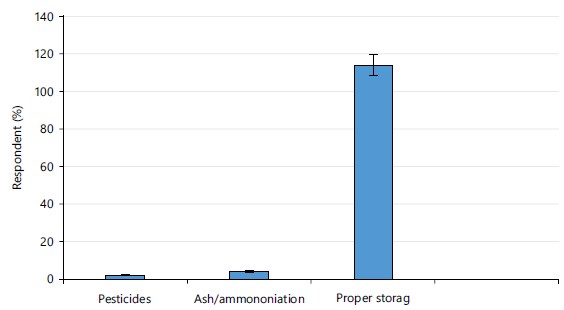
Post-harvest handling strategies for controlling diseases of cocoyam: One hundred and fifteen respondents adopted proper storage as a way of controlling post-harvest spoilage of cocoyam while one respondent mentioned pest control as a way of controlling diseases of cocoyam while only three respondents stated ash/ammoniation as a way of controlling post-harvest rot (Fig. 3).
Morphological characteristics of fungal isolates from cocoyam in Oji River Local Government Area: The fungal organisms associated with the rotten cocoyam cormels were identified based on colony appearance and morphology as presented in Table 4. A total of five fungal isolates were obtained from rotten cocoyams. The isolates include Mucor circinelloides, Fusarium oxysporum and Rhizopus stolonifer responsible for soft rots. The only dry rot producing mold was Penicillum cyclopium.
Frequency of occurrence of the isolated fungi/various disease conditions: The fungi pathogens that were constantly isolated from the rot-infested tissues of the cocoyam cormels include Aspergillus niger, Fusarium oxysporium, Mucor circinelloides, Rhizopus stolonifer and Penicillium expansum. The frequency of occurrence varied with different fungi associated with the rotten cocoyam cormels. The most frequently occurred were Penicillium expansum and Fusarium oxysporium with 32.3 and 20.8% of occurrence, respectively, while others had lower frequencies of occurrence ranging from 14.4 to 15.6% (Table 5). Dry rot had 37.5% while soft rot had 62.5% occurrence (Table 6).
| Table 4: | Colonial morphology and microscopic characterizations of fungi associated with cocoyam spoilage | |||
| Sample | Colonial characteristics | Microscopic characteristics | Suspected organisms (Specie) |
Rot type |
Pathogenicity |
| A | Fast growing pink and colony cotton |
Largest spores are circle shaped and may contain several cells |
Fusarium oxysporum | Soft |
+++ |
| B | On SDA, colonies are very fast growing, cottony to fluffy, white to yellow, becoming dark grey |
Sporangiosphores are hyaline, grey or brownish, globose to ellipsoidal and smooth-walled and erect with well-developed subtending columella |
Rhizopus stolonifer | Dry |
+++ |
| C | Yellow-green with white mycelia with loosely packed phialids |
Conidiophores and conidia columnar conidia heads were radiated to columnar |
Aspergillus niger | Soft |
++ |
| D | Colonies are velvety and fast growing with shades of green sometimes white |
Conidia are smooth and ellipsoidal. Mycelia are arranged irregularly with branches of various length |
Penicillium expansum | Dry |
+++ |
| E | On SDA, colonies were floccose, pale greyish-brown. Growth rate was rapid |
Sporangiophores were hyaline, erect, non-septate and branched sympodially and circinate |
Mucor circinelloides | Soft |
+++ |
| +++: Highly pathogenic and ++: Moderately pathogenic | |||||
| Table 5: | Frequency of occurrence of the isolated fungi | |||
| Organisms | Percentage frequency of occurrence |
| Fusarium oxysporum | 20.8 |
| Rhizopus stolonifer | 14.4 |
| Aspergillosis niger | 16.8 |
| Penicillium expansum | 32.3 |
| Mucor circinelloides | 15.6 |
| Table 6: | Frequency of occurrence of different disease conditions | |||
| Disease condition | Frequency of occurrence |
Occurrence (%) |
| Dry rot | 3 |
37.5 |
| Soft rot | 5 |
62.5 |
| Total | 8 |
100 |
Pathogenicity test: The pathogenicity test showed that all four test fungi (Rhizopus stolonifer, Fusarium oxysporum, Mucor circinelloides and Aspergillus niger) were pathogenic, hence cause rot in healthy cocoyam cormels after six days of inoculation. The most virulent among the four test fungi was Rhizopus stolonifer, with a rot incidence of 76%, followed by Aspergillus niger at 68% while the least virulent was Fusarium oxysporum with a rot incidence of 24%.
DISCUSSION
The socio-demographic data obtained from the survey showed that there are more female cocoyam farmers/sellers than males. This agreed with the documentation of Ezugwu15, who reported that there is a socio-cultural perception of cocoyam as a women’s crop in most parts of South-Eastern Nigeria. This was also in tandem with the findings of Ukeje et al.17, who reported that 65% of cocoyam farmers/traders are female. This study also showed that the age range of most cocoyam farmers/sellers is 41-45 years, this was in tandem with the findings of Apata et al.18, who stated that the average age of cocoyam farmers/traders is 45 years. The study of Apata et al.18 also supported the finding of this study that the majority of cocoyam farmers (58.7%) had post-primary education while 5% had no formal education. It was also confirmed from the inference of this study that the majority of the pre and post-harvest handlers of cocoyam all have knowledge of fungi diseases and can identify various symptoms of viral diseases in plants ranging from stunting, yellowing, necrosis etc. The main pre-harvest spoilage control method of cocoyam according to the study is crop rotation while the main post-harvest rot control method is proper storage. This was in tandem with the report of Arah et al.19.
Aspergillus niger, Fusarium oxysporum, Penicillium expansum, Rhizopus stolonifer and Mucor circinelloides were repeatedly isolated from the cocoyam cormels with symptoms of rot. There are scientific evidences that these organisms destroy root and tuber crops in storage20. This result supported the finding of many workers on other root and tuber crops15. The isolation of more than one pathogen from a particular cormel is responsible for the numerous infections whose combined effect may cause rapid maceration of the cormels, this was agreed with the results of Nwankiti and Gwa20 on yam. It has also been reported that fungi in most cases penetrate cocoyam cormels through natural opening such as stomata and wounds created during various cultural operations. Okigbo and Nmeka8 documented that root and tuber crops at time of harvest may already be infested by pathogens such as fungi and bacteria. The findings of this study agreed with the study conducted in Bhubaneswar City, India by Khatoon et al.21 who reported that, Aspergillus niger, Rhizopus spp., Penicillin spp and Geotrichum candidum were present in Colocasia esculenta tubers. Interestingly, Geotrichum candidum was not detected in this present study, this could be due to differences in environmental factors such as temperature and relative humidity across the study areas that favor the growth of this specific species. Similar species were reported by Frank and Kingsley22.
The ethnobotanical aspects of this study with reference to the socio-demographic data of the population and their perspective on the various pre and post-harvest handling practices of cocoyam have confirmed that most handlers have limited knowledge of fungi as causal agents of cocoyam rot hence the need for this study. The inference of this study is crucial in improving disease control because both information on the handling practices and pathogen responsible for cocoyam rot has been collacted and documented.
There was limited information on the pre and post-harvest handling practice of cocoyam in the study area but the findings of this study have added useful information on the perspective of cocoyam handlers within the study area. The inference of this study is of great importance to farmers, extension workers, the government and all other stakeholders involved in handling food samples.
This research has elucidated many facts about the methods of handling cocoyam by farmers and traders. Farmers in the study area need more information on the best methods of post and pre-harvest handling of cocoyam. On this note, the masses must be consistently sensitized on the best methods of handling cocoyam to avoid being exposed to pathogenic agents that induce rot. More research is also needed on the effective and environmentally friendly methods of controlling rot-inducing pathogens. It is also pertinent that government/relevant agencies fund research centred on a detailed study of fungal ecology and the development of relatively cheaper biological control and plant-based agents.
The limitations of the study include a paucity of funds that limited the research area to only Oji River LGA. This also limited the research to only the identification of pathogens responsible for rot without searching for the control measures of the pathogens. However, this has opened up a new area of research for further study. The cultural bias in the study area is another important limitation; most respondents have a belief/cultural perception that cocoyam is a women’s crop and women do not have control over land, labour and capital in the area.
Adequate measures should be taken to avoid inflicting injury to cocoyam before storage. Also, cocoyam with wounds should be separated from those without wounds to avoid contamination.
CONCLUSION
Cocoyam is one of the important root and tuber crops in Africa and consumed by a large population requires meticulous handling measures during planting, storage and across the various value addition stages. Various aseptic measures must be adopted to inhibit the contamination of cormels by pathogens and to prevent bioaccumulation of toxins produced by toxigenic fungi that may be detrimental to human and animal health. Sensitization and awareness campaigns should be carried out by the government and other relevant stakeholders, on good and effective handling techniques to minimize the rot of cocoyam and other root and tuber crops by pathogens. This no doubt will reduce the 40% annual loss of root and tuber crops due to rot and other forms of spoilage to achieve food safety and food security and in turn have a balanced economy.
SIGNIFICANCE STATEMENT
This study documents the various pre and post-harvest handling practices of cocoyam. It is on record that over 40% of root and tuber crops cultivated annually are lost to rot due to various improper cultural practices. This study seeks to gather information and document the various cultural practices that could predispose cocoyam corms and cornels to rot and to identify the fungal organisms responsible for their spoilage with the main objective of developing a safe, readily available and environment-friendly control measure.
REFERENCES
- Matikiti, A., J. Allemann, G. Kujeke, E. Gasura, T. Masekesa and I. Chabata, 2017. Nutritional composition of cocoyam (Colocasia esculenta), grown in Manicaland province in Zimbabwe. Asian J. Agric. Rural Dev., 7: 48-55.
- Adeosun, K.P., E.C. Amaechina and A.P. Nnaji, 2017. Determinants of households consumption preference for processed cocoyam in Enugu State, Nigeria. J. Dev. Agric. Econ., 9: 137-144.
- Opoku-Agyeman, M.O., S.O. Bennett-Lartey and C. Markwei, 2004. Agro-morphological and sensory characterization of cocoyam (Xanthosoma sagittifolium (L) (Schott) germplasm in Ghana. Ghana J. Agric. Sci., 37: 23-31.
- Falade, K.O. and C.A. Okafor, 2013. Physicochemical properties of five cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) starches. Food Hydrocolloids, 30: 173-181.
- Abdulrahman, S., A. Abdullahi and B. Muhammad, 2015. Analysis of constraints to cocoyam production in Kaduna State, Nigeria. J. Sci. Res. Rep., 6: 210-216.
- Gollifer, D.E. and R.H. Booth, 1973. Storage losses of taro corms in the British Solomon Islands protectorate. Ann. Appl. Biol., 73: 349-356.
- Hyde, K.D., J. Xu, S. Rapior, R. Jeewon and S. Lumyong et al., 2019. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diversity, 97: 1-136.
- Okigbo, R.N. and I.A. Nmeka, 2005. Control of yam tuber rot with leaf extracts of Xylopia aethiopica and Zingiber officinale. Afr. J. Biotechnol., 4: 804-807.
- Anukwuorji, C.A., C.M. Obianuju, R.O. Ezebo and C.L. Anuagasi, 2016. Antimicrobial effects of four plant extracts against post harvest spoilage fungi of yam (Dioscorea rotundata Poir). Int. J. Plant Soil Sci., 12.
- Anukwuorji, C.A., R.N. Okigbo, A.E. Chikwendu, C.L. Anuagasi and J.U. Anukwu, 2020. Heavy metals contamination of some food materials from markets in South Eastern Nigeria. Eur. J. Nutr. Food Saf., 12: 94-101.
- Agu, K.C., N.S. Awah, P.G. Sampson, M.O. Ikele and A.E. Mbachu et al., 2014. Identification and pathogenicity of rot-causing fungal pathogens associated with Xanthosoma sagittifolium spoilage in South Eastern Nigeria. Int. J. Agric. Innovations. Res., 2: 1155-1159.
- Chikwendu, A.E., R.N. Okigbo, C.A. Anukwuorji, J.U. Anukwu and H.N. Eze, 2020. Effects of some ethno medicinal plant extracts on Botryodiplodia theobromae the causal organism of Yam (Dioscorea rotundata Poir) rot. South Asian J. Res. Microbiol., 6: 17-2.6
- Raja, S., P. Subhashini and T. Thangaradjou, 2016. Differential methods of localisation of fungal endophytes in the seagrasses. Mycology, 7: 112-123.
- Okigbo, R.N., C.A. Anukwuorji and M.Z. Igweonu, 2014. Fungitoxic effect of Azadirachta indica and Vernonia amygdalina extracts on Aspergillus niger and Fusarium oxysporium the causal organisms of yam (Dioscorea rotundata) rot. Bioscientist J., 2: 70-86.
- Ezugwu, R.I., 2023. Isolation and identification of fungi associated with the spoilage of cocoyam corms. Int. J. Innovative Res. Dev., 12: 44-47.
- Lamini, S., E.W. Cornelius, F. Kusi, A. Danquah and P. Attamah et al., 2020. Prevalence, incidence and severity of a new root rot disease of cowpea caused by Macrophomina phaseolina (Tassi) Goid in Northern Ghana. West Afr. J. Appl. Ecol., 28: 140-154.
- Ukeje, B.A., M.E. Njoku, H.N. Anyaegbunam and P.N. Ajuka, 2018. Differentials in market participation among cocoyam farmers in Enugu State, Nigeria. Nigeria Agric. J., 49: 101-105.
- Apata, T.G., E.A. Henry, C. Jaiyeoba, O. Igbalajobi, K. Oni, O. Bamigboye and S. Bamisaye, 2021. Value chain analysis of cocoyam enterprise in Southwest Region, Nigeria. J. Austrian Soc. Agric. Econ., 17: 369-388.
- Arah, I.K., G.K. Ahorbo, E.K. Anku, E.K. Kumah and H. Amaglo, 2016. Postharvest handling practices and treatment methods for tomato handlers in developing countries: A mini review. Adv. Agric., 2016.
- Nwankiti, A.O. and V.I. Gwa, 2018. Evaluation of antagonistic effect of Trichoderma harzianum against Fusarium oxysporum causal agent of white yam (Dioscorearotundata poir) tuber rot. Trends Tech. Sci. Res., 1: 12-18.
- Khatoon, A., A. Mohapatra and K.B. Satapathy, 2016. Fungi associated with storage rots of Colocasia esculenta L. tubers in Bhubaneswar City, Odisha. Microbiol. Res. J. Int., 12.
- Frank, C.O. and C.A. Kingsley, 2014. Role of fungal rots in post-harvest storage losses in some Nigerian varieties of Dioscorea species. Microbiol. Res. J. Int., 4: 343-350.
How to Cite this paper?
APA-7 Style
Azubuike,
A.C., Anthonia,
N.A., Okwudili,
O.G., Chimaobi,
N.J. (2023). Post-Harvest Handling Survey Report on Cocoyam [Colocasia esculenta (L.) Schott] in Oji-River, Enugu State, Nigeria. Asian Journal of Biological Sciences, 16(4), 590-599. https://doi.org/10.3923/ajbs.2023.590.599
ACS Style
Azubuike,
A.C.; Anthonia,
N.A.; Okwudili,
O.G.; Chimaobi,
N.J. Post-Harvest Handling Survey Report on Cocoyam [Colocasia esculenta (L.) Schott] in Oji-River, Enugu State, Nigeria. Asian J. Biol. Sci 2023, 16, 590-599. https://doi.org/10.3923/ajbs.2023.590.599
AMA Style
Azubuike
AC, Anthonia
NA, Okwudili
OG, Chimaobi
NJ. Post-Harvest Handling Survey Report on Cocoyam [Colocasia esculenta (L.) Schott] in Oji-River, Enugu State, Nigeria. Asian Journal of Biological Sciences. 2023; 16(4): 590-599. https://doi.org/10.3923/ajbs.2023.590.599
Chicago/Turabian Style
Azubuike, Anukwuorji, Chidozie, Nwokolo Amarachukwu Anthonia, Ogbuozobe Gabriel Okwudili, and Ndubuisi Joel Chimaobi.
2023. "Post-Harvest Handling Survey Report on Cocoyam [Colocasia esculenta (L.) Schott] in Oji-River, Enugu State, Nigeria" Asian Journal of Biological Sciences 16, no. 4: 590-599. https://doi.org/10.3923/ajbs.2023.590.599

This work is licensed under a Creative Commons Attribution 4.0 International License.



