Growth Parameters and Alliance Between Selected Morphometric Features of Schilbe mystus in Asejire Reservoir, Nigeria
| Received 29 Sep, 2023 |
Accepted 10 Nov, 2023 |
Published 31 Dec, 2023 |
Background and Objective: Captive rearing of fish requires up-to-date information on key biological attributes such as growth parameters, size structure and other morphometric indices. This study thus investigates the morphometric characteristics of some key aging structures and growth parameters of Schilbe mystusin Asejire Reservoir, Nigeria. Materials and Methods: The 306 samples were obtained and weighed. Total length (TL), cleithra length (CL) and otolith height (OH) were measured. Length at first maturity (Lm), optimum length (Lopt), asymptotic length (L∞), growth co-efficient (K) and overall growth performance index (Ø) for each sex were calculated using von Bertalanffy growth model. Data obtained were analyzed using descriptive statistics, chi-square and multiple linear regression. All statistical analyses were considered at a significant level of 5% (p<0.05). Results: The TL of the fish ranged from 11.5-22.0 cm and the body weight ranged from 8.00-74.00 g. The Log W = -1.5836+2.7015 log L described the length-weight relationship. The fish exhibited negative allometric growth (K = 1.195±0.19) while length frequency distribution showed one modal peak (14-17 cm). The growth parameters were: Lm = 12.5, 14.1 cm, Lopt = 12.3, 14.1, L∞ = 20.4, 23.2 cm, K = 0.26, 0.05 and Ø = 2.03, 1.43 for male and female, respectively. The regression equation describes the relationship between weight, fish length and otolith, opercula and cleithra dimensions of fish. Conclusion: The study suggested that otolith dimensions’ increase as fish length increases and therefore, otolith growth correlated with fish growth. The female S. mystus grows bigger and are more preponderance in Asejire Reservoir than male.
| Copyright © 2023 Kazeem et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The freshwater Nile Silver Schilbeid Catfish, Schilbe mystus (L), family Schilbeidae, is widely distributed in West Africa1 and constituted one of the most dominant fish species in Nigeria inland waters. It is an inland foraging fish species which move in group in the middle of water with vegetation near the water’s edge or below the water surface. This nocturnal species spends its entire life in freshwater but usually travel to flood waters for breeding during rainfall2. It is highly relish among low-income earners due to its good quality and tasty white flesh3 and sold in the aquarium trade. Schilbe mystus is an omnivore which grows up to 70 cm in length and 250 g in weight4. There is a marked sexual dimorphism in the species and both male and female gonads mature at different age depending on the systems5.
Growth and dimension of morphometric characteristic studies are of practical importance for describing the status of fish population and for predicting the potential of fisheries6. Studying age, growth and morphometric characteristics of fishes is necessary for their sustainable exploitation, preservation and management. In fisheries assessment, important information such as weight from a length, ontogenic allometric changes and condition index are used to predict the potential yield and to determine the appropriate size at capture for sustainability7. Merino et al.8 pinpointed that length and weight data provide statistics that are cornerstones in the foundation of fishery research and management. In addition, hard parts such as scales, otoliths, spines, opercular bones, fin rays and vertebrae are very useful for age and growth studies, with a focus on inputs for stock assessments9 and to produce basic information about growth parameters of fish stocks.
There are many works on age and growth of freshwater fish species in Nigerian inland waters9-11, but there is a need to update information on age and growth of Schilbe mystus in Asejire Lake, Nigeria12. This study therefore assessed growth parameters, length-weight relationship and condition factor and morphometric characteristics of hard parts of S. mystus in Asejire Lake, Nigeria.
MATERIALS AND METHODS
Study area: This study was conducted in Asejire Reservoir between January and July, 2018 covering both dry and wet seasons in the area. The lake is manmade and constructed on Osun River which is the main river in the basin of Southwestern Nigeria. It is situated around 7°21'30", 7°21'50"N, 4°07'30" and 4°08'10"E, at an altitude of 137 m above sea level. The lake is Y-shaped with two unequal arms: The longer arm is River Osun, while the shorter one is River Oba13. The basin is inundated by floodwater of the River Oshun and its main tributary River Oba. The lake has a surface area of 24 km–2, a maximum flood elevation of 152.4 m, a catchment and an impounded area of 7,800 km–² and 2,342 ha, respectively. Fishing activity is prominent in the Reservoir.
Sample collection: Samples (n = 306) were collected using cast nets and surface gill nets over 8 months from December, 2018 and July, 2019. Species identification was performed with the aid of reference materials3. Specimens were conveyed in ice-packed bags to the wet laboratory, Department of Aquaculture and Fisheries Management, University of Ibadan for further analysis. All fish were sexed, weighted to the nearest 0.01 g and the total length measured to the nearest 0.1 cm.
Length weight relationship (LWR) and condition factor: The relationship between different length types (standard length and total length) and body weight of S. mystus specimens was expressed by the following equation described by Le Cren14:
where, W is total weight, L is length and (a and b) is constants whose values were estimated by the least square method. Fulton’s condition factor (K) was c alculated based on Le Cren14 using the equation:
K |
= |
Condition factor |
|
W |
= |
Weight of fish (g) |
|
L |
= |
Length of fish (cm) |
The correlation coefficient (R2) was estimated to determine the degree of relationship between the length and weight of the samples.
Opercula extraction and measurement: The opercula bones were removed from the fish by prying them off the head with a blunt probe and ripping them off as close to the skull as possible, being careful not to crack the opercula bone. The opercula bones were dipped in a petri dish containing hydrogen peroxide for about two minutes to remove the surrounding tissues and then washed with clean water, air-dried and later examined under a stereoscope15.
Otoliths extraction and measurement: Otoliths were removed and processed following the procedure of Gebremedhin et al.16. Otolith images were shot using Leica DM IRB stereo microscope (Microscope central, Bustleton Pike, Pennsylvania and United States of America) with camera system for size dimension. Otolith length and height were calculated to the nearest 0.01 mm. The otolith length was delineated from the midpoint of the rostrum at point A through the primordium to the posterior edge at point B, while height was measured vertically to the length passing through the primordium17.
Cleithra extraction and dimension: Cleithera is the paired bones of the pectoral girdle that form the frame of the body wall directly posterior to the opercula cavity18. Cleithrum was removed and processed using the method of Gebremedhin et al.16. The structures were cleaned, placed in a vial and dried before measuring. The relationship between the otolith size (length-OL and height-OH), opercula size (OPL and OPH), CL and fish size (total length-TL and total weight-TW) were determined using a power regression model between various measurements19. The agreement between the models and the data was verified with the coefficient of determination (r2).
Growth parameters: Growth was characterized using the von Bertalanffy growth function, fitted to size-at-age data using standard nonlinear optimization methods. The von Bertalanffy growth function is explained as (von Bertalanffy20):
where, Lt is length at age t, L∞ is the asymptotic length, K is the growth coefficient and t0 is the hypothetical age at which length is zero.
The formula of Pauly et al.21 was used to compute the index of overall growth performance:
where, K and L∞ are parameters of von Bertalanffy growth function.
Statistical analysis: Data obtained were analyzed using descriptive statistics, chi-square and multiple linear regression in Statistical Package for Social Sciences (SPSS for Windows version 20). All statistical analyses were considered at a significant level of 5% (p<0.05).
Ethical consideration: The authors confirm that the ethical policies noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval was received. The US National Research Council's guidelines for the Care and Use of Laboratory Animals were followed.
RESULTS
Morphometric parameters of Schilbe mystus: Schilbe mystus in Asejire Reservoir had a mean TL value of 16.68±1.41 cm with a Coefficient of Variation of 8.4 (Table 1). The mean value of SL was 4.15±1.22 cm while weight has a mean value of 34.41±9.04 g and a Coefficient Variation of 26.28. The mean length of 0.12±0.02 cm with a Coefficient Variation of 18.83 was obtained for the Otolith. Otolith height had a mean value of 0.08±3.66 cm and Coefficient Variation of 52.29. Opercula length had a mean value of 1.25±1.22 cm while the mean value of Opercula height was 0.96±2.25 cm. Cleithra length had a mean value of 1.68±0.21 cm with Coefficient Variation of 12.34. The TL and SL ranged from 11.5 to 22.0 cm and 10.0 to 18.0 cm, respectively. There was no significant difference in each of the parameters taken between sexes from the population at p<0.05.
Morphometric parameters across sexes of Schilbemystus in Asejire Reservoir: The mean TL value of 16.65±0.09 and 16.89±0.18 cm was obtained for females and males, respectively. The effect of the sex was also not significant on the Standard length with mean value of 14.11±0.08 and 14.48±0.16.00 cm for females and male, respectively. The Otolith length has a mean value of 0.12±0.001 and 0.12±0.001 cm for females and males, respectively. The effect of sex was also not significant on the Otolith height. The mean value obtained for Opercula length for both sexes were 1.27±0.23 and 1.08±0.02 cm, for females and males, respectively. The effect of the sex was insignificant on the Opercula height for both sexes (0.99±0.27 and 0.75±0.02 cm for female and male, respectively). The Cleithra lengths for females and males, respectively were 1.68±0.01 and 1.69±0.03 cm.
Correlation matrix and regression coefficient for morphometric parameters of Schilbe mystus: The correlation matrix for the morphometric of Schilbe mystus in Asejire Reservoir is as shown in Table 2. The values obtained for morphometric parameters examined were significantly correlated (p<0.05) with each other except OH and OPH. The SL, WT, OL and CL show positive correlation with TL, while OPL and OPH reveal no significant negative correlation (p>0.05). The WT, OL, OPH and CL were markedly correlated with SL but OH and OPL were slightly correlated. The OPL had significant negative correlation with SL. The OL and CL had a significant positive correlation with WT while OPL showed slightly negative correlation. Table 3 depicts the regression coefficient for morphometric parameters of Schilbe mystus in Asejire. Total length can explain the weight of the fish in the Reservoir by 78% as revealed in the coefficient of determination (R2) which is highly significant (p<0.05). Standard length can predict the weight of the fish up to 65% as indicated by R2 the value (0.65). The Otolith length (OL) can predict the weight of the fish by 24% while Otolith height can only explain the weight of the fish by 6%.
| Table 1: | Morphometric parameters of Schilbe mystus from Asejire Reservoir | |||
Range |
|||||||
| Variable | N |
Mean |
Standard deviation |
Standard error |
Min |
Max |
CV |
| TL | 306 |
16.7 |
1.4 |
0.08 |
11.5 |
22 |
8.43 |
| SL | 306 |
4.15 |
1.2 |
0.07 |
10 |
18 |
8.63 |
| WT | 306 |
34.4 |
9.04 |
0.52 |
8.1 |
74 |
26.28 |
| OL | 306 |
0.12 |
0.02 |
0 |
0.08 |
0.35 |
18.83 |
| OH | 306 |
0.08 |
3.66 |
0 |
0.06 |
0.8 |
52.29 |
| OPL | 306 |
1.25 |
1.22 |
0.21 |
0.08 |
65 |
23.93 |
| OPH | 306 |
0.96 |
2.25 |
0.24 |
0.06 |
75 |
42.26 |
| CL | 306 |
1.68 |
0.21 |
0.02 |
0.6 |
2.3 |
12.34 |
| TL: Total length (cm), SL: Standard length (cm), WT: Weight of fish (g), OL: Otolith length (cm), OH: Otolith height (cm), OPL: Opercular length (cm), OPH: Opercular height (cm), CL: Cleithra length (cm), CV: Coefficient of variation (%), Min: Minimum and Max: Maximum | |||||||
| Table 2: | Correlation matrix for morphometric parameters of Schilbe mystus in Asejire Reservoir | |||
| Parameters | TL | SL | WT | OL | OH | OPL | OPH | CL |
| 1 | 0.91796 | 0.88218 | 0.32996 | 0.06042 | -0.18874 | -0.01073 | 0.60458 | |
| TL | <.0001 | <.0001 | <.0001 | 0.2921 | 0.0009 | 0.8517 | <.0001 | |
| 306 | 306 | 306 | 306 | 305 | 306 | 306 | ||
| 1 | 0.80542 | 0.3276 | 0.05893 | -0.17323 | 0.03209 | 0.55839 | ||
| SL | <.0001 | <.0001 | 0.3042 | 0.0024 | 0.576 | <.0001 | ||
| 306 | 306 | 306 | 305 | 306 | 306 | |||
| 1 | 0.3472 | 0.0816 | -0.14239 | 0.00169 | 0.63491 | |||
| WT | <.0001 | 0.1545 | 0.0128 | 0.9765 | <.0001 | |||
| 306 | 306 | 305 | 306 | 306 | ||||
| 1 | 0.22727 | -0.09289 | -0.02079 | 0.1902 | ||||
| OL | <.0001 | 0.1054 | 0.7172 | 0.0008 | ||||
| 306 | 305 | 306 | 306 | |||||
| 1 | -0.02609 | -0.01561 | 0.08467 | |||||
| OH | 0.6499 | 0.7857 | 0.1395 | |||||
| 305 | 306 | 306 | ||||||
| 1 | -0.00543 | -0.14279 | ||||||
| OPL | 0.9248 | 0.0126 | ||||||
| 305 | 305 | |||||||
| 1 | 0.0315 | |||||||
| OPH | 0.5831 | |||||||
| 306 | ||||||||
| 1 | ||||||||
| CL | 306 | |||||||
| TL: Total length (cm), SL: Standard length (cm), WT: Weight of fish (g), OL: Otolith length (cm), OH: Otolith height (cm), OPL: Opercular length (cm), OPH: Opercular height (cm), CL: Cleithra length (cm) and Significant at 5% level (p<0.05) | ||||||||
| Table 3: | Regression Coefficient for Morphometric Parameter of Schilbe mystus in Asejire Reservoir | |||
| Parameters | a |
b |
r2 |
MSE (b) |
p-value |
Equation |
| SL | 0.18 |
0.9 |
0.86 |
0.0002 |
<0.0001 |
TL = 0.18+0.9 SL |
| WT | 0.8 |
0.27 |
0.81 |
0.00027 |
<0.0001 |
TL = 1.51+0.27 WT |
| OL | 1.51 |
0.32 |
0.24 |
0.0012 |
<0.0001 |
TL = 1.51+0.32 OL |
| OH | 1.34 |
0.11 |
0.06 |
0.0013 |
<0.0001 |
TL = 1.34+0.11 OH |
| OPL | 1.2 |
0.03 |
0.01 |
0.4284 |
<0.034 |
TL = 1.20+0.03 OPL |
| OPH | 1.23 |
0.07 |
0.07 |
0.403 |
<0.0001 |
TL = 1.23+0.07 OPH |
| CL | 1.15 |
0.31 |
0.3 |
0.001 |
<0.0001 |
TL = 1.15+0.31 CL |
| ‘a’ intercept, ‘b’ slope, ‘r2’ coefficient of determination, ‘MSE(b)’ Mean standard error of the slope, TL: Total length (cm), SL: Standard length (cm), WT: Weight of fish (g), OL: Otolith length (cm), OH: Otolith height (cm), OPL: Opercular length (cm), OPH: Opercular height (cm), CL: Cleithra length (cm) and Significant at 5% level (p<0.05) | ||||||
Opercula length can explain the weight of the fish by 10% while Opercula height shows a significant negative correlation. Cleithra length can explain the weight of the fish by 40%. The TL, OH and CL can best predict the weight of the fish in Asejire Reservoir with significant p<0.05 following the stepwise Multiple regression.
The regression equation is given as:
This implies that the contributions of these 3 parameters are best used in predicting the age of Schilbe mystus in Asejire Reservoir. The SL, WT and OPH can best be used in predicting the total length of Schilbe mystus in Asejire Reservoir with multiple regression equation given as:
with high positive correlation coefficient of 0.86, 0.91 and 0.91, respectively.
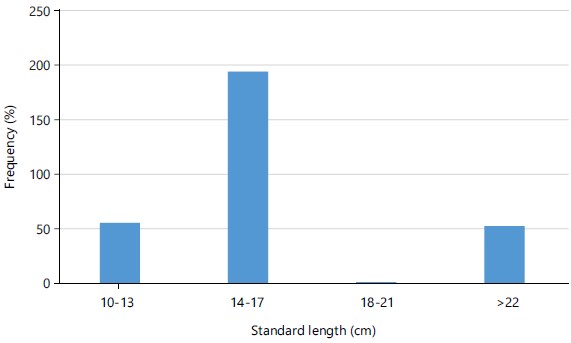
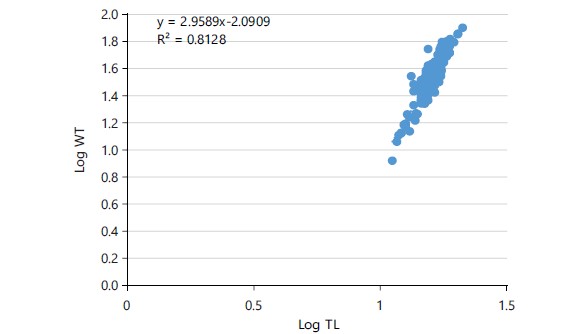
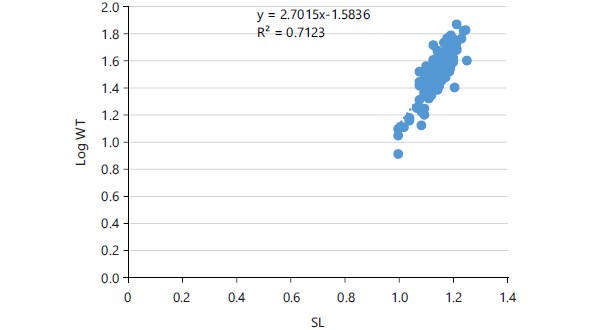
Length frequency distribution and length-weight relationship: The total length recorded for Schilbe mystus from Asejire Reservoir varies from 11.0 to 22.0 cm while the Standard-length ranges from 10.0 and 18.9 cm, respectively. The length frequency distribution of this species is presented in Fig. 1. The weight of 306 samples of Schilbe mystus ranges from 8.1 to 74.0 g. The length, as independent variable (X) was regressed over the weight as dependent variable (Y) to calculate the values of “a” and “b” forming the regression equation (Fig. 2-3). The values of “a” and “b” recorded were -1.5836 and 2.7015, respectively. The regression equation obtained was:
The coefficient of determination and correlation coefficient was calculated to measure the degree of relationship between length and weight of Schilbe mystus. The value of 0.7123 was recorded as coefficient of determination (r2) and 0.81 was recorded as correlation (r). The marked correlation was significant (p<0.05) as shown in Table 3.
|
|
|
| Table 4: | Growth parameters of Schilbe mystus from Asejire Reservoir | |||
| Variable | Male |
Female |
Combined sex |
| Lmax | 19.3 |
22 |
22 |
| Lopt | 12.3 |
14.1 |
14.1 |
| Lm | 12.5 |
14 |
14 |
| Nms | 32 |
274 |
287 |
| L’ | 0 |
0 |
17 |
| Lmean | 16.89±0.18 |
16.65±0.09 |
16.68±1.41 |
| L∞ | 20.4 |
23.2 |
23.2 |
| L1 | 16 |
12.5 |
16.5 |
| L2 | 17 |
13 |
18.5 |
| t0 | 1 |
1 |
1 |
| K | 0.26 |
0.05 |
0.35 |
| Ø | 2.03 |
1.43 |
2.3 |
| Lmax: Maximum length, Lopt: Length with optimum yield, Lm: Length at first maturity, Nms: Number of mature specimens, L’Smallest fully selected length class, Lmean: Mean length in the sample, L∞: Asymptotic length, L1: Length associated with first peak, L2: Length associated with first peak, t0: Time difference between L1 and L2 and growth coefficient (K) and Ø: Overall growth performance index | |||
Growth parameters of Schilbe mystus from Asejire Reservoir: Table 4 contains growth parameters of Schilbe mystus in Asejire Lake. Length at first maturity (Lm) was 14 cm and length with optimum yield (Lopt) was within the range of 11.5 and 14.1, respectively. The growth parameters obtained with length frequency analysis wizard were:
| Lm | = | 12.5, 14.0 and 14.0 | |
| Lopt | = | 12.3, 14.1 and 14.1 | |
| L∞ | = | 20.4, 23.2 and 23.2 | |
| K | = | 0.26, 0.05 and 0.35 |
for male, female and combined sex, respectively (Table 4). Also, the value for the hypothetical age was one year. The von Bertalanffy growth model for the species is described as:
Overall growth performance index (Ø) was found to be 2.30, 2.03 and 1.43 total length for combined sex, male and female, respectively.
DISCUSSION
In the present study, the length-weight distributions of Schilbe mystus from Asejire Lake showed considerably large variations in fish sizes indicating an efficient gill net operation. The 306 specimens collected were mostly comprises of large samples and thus were considered reasonably representative and reliable. The smallest sample size corresponded to the infrequent species and the largest samples belonged to those which were frequently encountered in large numbers. Similar observation was reported by Olanrewaju et al.22 for seven commercially important freshwater fishes in Asejire Lake, Nigeria. McCravy23, however, noted that species diversity is highly determined by sampling size and sampling methods including type of gear, screen size and sorting technique. The sample size obtained from this study was like that of Khan et al.24, who studied the length-weight relationships (LWR) and condition factors six fish species in Atbara River and Khash el-Girba Reservoir, respectively. The overall species sampled had more females (274) than males (32), an indication of female dominant population. This was consistent with work of Kareem et al.25 and Dan-Kishiya26, who reported the female dominance population in Erelu Lake, Oyo and Lower Usuma Reservoir in Abuja, respectively. The result showed that females were longer and heavier than males for all sampled species. Kareem et al.25 also reported longer and heavier females in the sample population of Chrysichythys nigrodigitatus and Schilbe mystus. Le Cren14 however, implied that females are heavier than males of the same length probably because of difference in fatness and gonadal development.
The growth exponential value “b” in combined sexes of S. mystus was 2.7 indicating negative allometric growth. Growth is said to be isometric when there is no change in body proportion and specific gravity, while allometric growth is characterized with dimensional change with growth. The “b” value calculated for S. mystus suggested that a unit increase in log of weight will correspond to about three times increase in log of Standard length. Famoofo and Abdul27 reported positive allometric growth (3.012±0.14) pattern for Schilbe uranoscopus in Lekki Lagoon, which contrasts with present findings. Results in this study, however, showed similar trend with earlier studies involving S. mystus from different water bodies in Nigeria as reported by Dan-kishiya26 in Lower Usuma Reservoir and Kareem et al.25 in Erelu Lake. The condition factor (2.11±0.59) in combined sexes of S. mystus corresponded with the findings of Dan-Kishiya26 in lower Usuma Reservoir and Kareem et al.25 in Erelu Reservoir, Nigeria. This result indicates good environmental condition for the fish well-being, growth and survival in Asejire Reservoir.
The findings revealed that otolith dimensions’ increase as fish length increases, therefore otolith growth can be correlated with fish growth. However, the otolith length had more correlation to the fish length than otolith height and other parameters. These results coincided with the report of Gaughan et al.28 on Sardinops sagax from the South Coast of Western Australia. In this study, Otolith dimensions, Opercula dimension and Cleithra length was linearly correlated to total fish length. Moreover, relationship between Total length of fish and Standard length (SL), Weight (WT), Otolith length (OL) and Cleithra Length (CL) showed highest positive correlation. This implies that increase in Otolith Length, Weight of the fish (WT) and Cleithra length results in increasing total length of the fish with Total length as a dependent variable. This was similar to the reports on Sardina pilchardus from Adriatic Sea, Croatia29, Sardinops sagax from North America18 and S. sagax from Australia. Campana and Casselman30 also identified significant relationship between growth rates and otolith shape in Icelandic cod, Gadus morhua. Otolith morphology has become an essential mechanism regularly applied to ascertain fish population structure and yearly changes. Further, Kristoffersen31 revealed that rapid growth rates are correlated to smaller otolith sizes corresponding to fish length in various species. One of the factors considered to be affecting growth and otolith morphology is feeding condition32. In addition, Høie et al.33, who studied maternal, paternal and temperature effects on otolith size of young herring (Clupea harengus L.) larvae found out that temperature affect otolith size in cultured fish. However, Begg and Brown34 contemplate that temperature have effect on otolith growth in haddock fishery.
Fitting the seasonalised von Bertalanffy growth function to our length/frequency data gives the biological date needed for growth parameters estimation. Length frequency data offer an important record from which invaluable information concerning the recent life history of the fish could be extracted35. The computed value of L∞ (23.2 cm) for combined sex of Schilbe mystus in Asejire Reservoir differ from values of 28.5cm and 28.7cm in Asejire and Eleyele Reservoir, respectively12. Also, Abdellatif et al.36 obtained L∞ = 27.5 crn for Schilbe intermedius in Cross River. However, larger sizes (L∞ = 35.43cm) were obtained for Chrysichthys auratus in Lake Nasser, Egypt36. The modal class is 14-17 which had the highest frequency and accounted for 64.05% of total specimen examined indicating that most of the catches are above the size at first maturity. The overall growth performance (Ø) for Schilbe mystus (2.30) in this study correspond to the value reported by Ayoade12 for the same species in Asejire (2.62) and Oyan (2.51) Lakes in Southwestern Nigeria. El-Kasheif et al.37 however, obtained higher growth performance indices in males (2.689), females (2.692) and combined sexes (2.709) of Synodontis schall in River Nile, Gizza sector in Egypt. El-Kasheif et al.37 also recorded somewhat higher growth performance index of 2.55 in Chrysichthys auratus from Lake Nasser, Egypt. Growth performance indices are indicators to evaluate and compare fish species from the same and/or different waterbodies.
This study revealed that the size at first maturity (L50) for Schilbe mystus in Asejire Lake is 14.0 cm, which implies that the fish below this size should not be harvested for good reproductive cycles and recruitment of the stock. Similarly, the condition factor of the species reveals good general wellbeing and fitness in Asejire Lake, which is an indication of good environmental condition. The information became highly helpful for managing the S. mystus stock for sustainable use, which enhanced the economic and social status of those who depended on the stock. The limitation of this study is essentially low sample size due to shortage of funds. Thus, more fund to pursue fisheries management research in inland water for sustainable fish supply and robust livelihood for artisanal fisher folks in the areas.
CONCLUSION
The current study suggested that otolith growth is correlated with fish growth, since otolith dimensions increase as fish length increases. African Butterfish Schilbe mystus exhibited negative allometric growth while the length at first maturity, optimum length and length at infinity and total length, for males and females, respectively. The growth model indicated that Schilbe mystus belongs to one size group (14-17 cm). The female S. mystus grows bigger and is more preponderance in Asejire Reservoir than male. The information becomes very useful for S. mystus stock management for sustainable utilization, which strengthens the social and economic standard of the people depending on the stock.
SIGNIFICANCE STATEMENT
The study is significant for effective management of Schilbe mystus populations in Asejire Reservoir, Nigeria. The study revealed the relationship between the otolith growth and the size of S. mystus, which is important for the rational exploitation of this fish stock. It also provides information on length-weight relationship and condition factor to describe the present status of the stock. The study has therefore provided crucial growth parameters insight for managing S. mystus.
REFERENCES
- Ayoade, A.A., 2012. The carcass chemical composition of the African butter catfish (Schilbe mystus) from two tropical Manmade Lakes, Southwestern Nigeria. Zool. Ecol., 22: 20-22.
- Idodo-Umeh, G., 2015. The feeding ecology of schilbeid catfishes in River Ase, Niger Delta, Southern Nigeria. Trop. Freshwater Biol., 24: 45-62.
- Reed, W., J. Burchad, A.J. Hopson, J. Jennes and I. Yaro, 1967. Fish and Fisheries of Northern Nigeria. Gaskiya Corporation, Northern Nigeria, Zaria, Pages: 226.
- Kareem, K., N. Olanrewaju and B. Igbaro, 2021. Growth pattern, diet and tropical niche breadth of the Nile silver catfish, Schilbe mystus (Linne 1758) in Asejire Lake, Southwestern, Nigeria. Egypt. J. Aquat. Biol. Fish., 25: 683-693.
- Breder, C.M. and D.E. Rosen, 1966. Modes of Reproduction in Fishes. T.F.H. Publications, Neptune City, pp: 941.
- Flinn, S.A. and S.R. Midway, 2021. Trends in growth modeling in fisheries science. Fishes, 6.
- Mello, F.T., C. Iglesias, A.I. Borthagaray, N. Mazzeo, J. Vilches, D. Larrea and R. Ballabio, 2006. Ontogenetic allometric coefficient changes: Implications of diet shift and morphometric traits in Hoplias malabaricus (Bloch) (Characiforme, Erythrinidae). J. Fish Biol., 69: 1770-1778.
- Merino, G., H. Murua, J. Santiago, H. Arrizabalaga and V. Restrepo, 2020. Characterization, communication, and management of uncertainty in Tuna fisheries. Sustainability, 12.
- Akombo, P.M., E.T. Akange and J.I. Atile, 2015. Age and growth of catfish Synodontis schall, (Bloch and Schneider, 1801) in the Lower Benue River, at Makurdi, Nigeria. Int. J. Fish. Aquat. Stud., 2: 184-190.
- Okogwu, O.I., 2011. Age, growth and mortality of Clarias gariepinus (Siluriformes: Clariidae) in the mid-cross river-floodplain ecosystem, Nigeria. Rev. Biol. Trop., 59: 1707-1716.
- Adeyemi, S.O. and P.M. Akombo, 2012. Age and growth of dominant cichlids in Gbedikere Lake, Kogi Statr, Nigeria. Anim. Res. Int., 9: 1497-1501.
- Adedolapo, A., 2007. Age and growth of the African butter catfish, Schilbe mystus (Linnaeus, 1758) in Asejire and Oyan Lakes, South-Western Nigeria. J. Fish. Aquat. Sci., 2: 110-119.
- Utete, B. and B.T. Fregene, 2020. Assessing the spatial and temporal variability and related environmental risks of toxic metals in Lake Asejire, South-Western Nigeria. Sci. Afr., 7.
- Le Cren, E.D., 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol., 20: 201-219.
- Campana, S.E. and J.D. Neilson, 1985. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci., 42: 1014-1032.
- Gebremedhin, S., K. Bekaert, A. Getahun, S. Bruneel, W. Anteneh, P. Goethals and E. Torreele, 2019. Comparison of otolith readability and reproducibility of counts of translucent zones using different otolith preparation methods for four endemic Labeobarbus species in Lake Tana, Ethiopia. Water, 11.
- Bermejo, S., 2007. Fish age classification based on length, weight, sex and otolith morphological features. Fish. Res., 84: 270-274.
- Schmitt, D.N. and W.A. Hubert, 1982. Comparison of cleithra and scales for age and growth analysis of yellow perch. Progressive Fish-Culturist, 44: 87-88.
- Zar, J.H., 2010. Biostatistical Analysis. 5th Edn., Pearson Prentice-Hall, New Jersey, United States, ISBN: 9780131008465, Pages: 944.
- von Bertalanffy, L., 1957. Quantitative laws in metabolism and growth. Q. Rev. Biol., 32: 217-231.
- Pauly, D., A.W. Trites, E. Capuli and V. Christensen, 1998. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci., 55: 467-481.
- Olanrewaju, A.N., O.K. Kareem, M.A. Akintunde and A. Jenyo-Oni, 2016. Studies on length-weight relationship and condition factor of seven commercially important freshwater fish species of Asejire Lake, Ibadan, Nigeria. Afr. J. Fish. Aquat. Resour. Manage., 1: 21-29.
- McCravy, K.W., 2018. A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects, 9.
- Khan, S., M.A. Khan, K. Miyan and M. Mubark, 2011. Length-weight relationship of nine freshwater teleosts collected from river Ganga, India. Int. J. Zool. Res., 7: 401-405.
- Kareem, O.K., A.N. Olanrewaju and O. Orisasona, 2015. Length-weight relationship and condition factor of Chrysichythys nigrodigitatus and Schilbe mystus in Erelu Lake, Oyo State, Nigeria. J. Fish. Livest. Prod., 3.
- Dan-Kishiya, A.S., 2013. Length-weight relationship and condition factor of five fish species from a tropical water supply reservoir in Abuja, Nigeria. Am. J. Res. Commun., 1: 175-187.
- Famoofo, O.O. and W.O. Abdul, 2020. Biometry, condition factors and length-weight relationships of sixteen fish species in Iwopin fresh-water ecotype of Lekki Lagoon, Ogun State, Southwest Nigeria. Heliyon, 6.
- Gaughan, D.J., R.W. Mitchell and S.J. Blight, 2000. Impact of mortality, possibly due to herpesvirus, on pilchard Sardinops sagax stocks along the south coast of Western Australia in 1998-99. Mar. Freshwater Res., 51: 601-612.
- Zorica, B., G. Sinovčić and V.Č. Keč, 2010. Preliminary data on the study of otolith morphology of five pelagic fish species from the Adriatic Sea (Croatia). Acta Adriatica, 51: 89-96.
- Campana, S.E. and J.M. Casselman, 1993. Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci., 50: 1062-1083.
- Kristoffersen, J.B., 2007. Growth rate and relative otolith size in populations of adult Müller’s pearlside Maurolicus muelleri. J. Fish Biol., 71: 1317-1330.
- Hüssy, K., 2008. Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol., 364: 35-41.
- Høie, H., A. Folkvord and A. Johannessen, 1999. Maternal, paternal and temperature effects on otolith size of young herring (Clupea harengus L.) larvae. J. Exp. Mar. Biol. Ecol., 234: 167-184.
- Begg, G.A. and R.W. Brown, 2000. Stock identification of haddock Melanogrammus aeglefinus on georges bank based on otolith shape analysis. Trans. Am. Fish. Soc., 129: 935-945.
- Etim, L., P.E. Lebo and R.P. King, 1999. The dynamics of an exploited population of a siluroid catfish (Schilbe intermedius Ruppell, 1832) in the Cross River, Nigeria. Fisheries Res., 40: 295-307.
- Abdellatif, M., E. Mohammed-AbdAllah, K.Y. AbouelFadl and A.G.M. Osman, 2022. Age and growth of Chrysichthys auratus (Geoffroy 1809) (Family: Claroteidae) from Lake Nasser, Egypt. Egypt. J. Aquat. Res., 48: 417-424.
- El-Kasheif, M.A., M.M.N. Authman and S.A. Ibrahim, 2012. Environmental studies on Synodontis schall (Bloch and Schneider, 1801) (Pisces: Siluriformes: Mochokidae) in the River Nile at Gizza Sector, Egypt: Biological aspects and population dynamics. J. Fish. Aquat. Sci., 7: 104-133.
How to Cite this paper?
APA-7 Style
Kazeem,
K.O., Nurudeen,
O.A., Victor,
O.F., Seun,
A.T. (2023). Growth Parameters and Alliance Between Selected Morphometric Features of Schilbe mystus in Asejire Reservoir, Nigeria. Asian Journal of Biological Sciences, 16(4), 555-565. https://doi.org/10.3923/ajbs.2023.555.565
ACS Style
Kazeem,
K.O.; Nurudeen,
O.A.; Victor,
O.F.; Seun,
A.T. Growth Parameters and Alliance Between Selected Morphometric Features of Schilbe mystus in Asejire Reservoir, Nigeria. Asian J. Biol. Sci 2023, 16, 555-565. https://doi.org/10.3923/ajbs.2023.555.565
AMA Style
Kazeem
KO, Nurudeen
OA, Victor
OF, Seun
AT. Growth Parameters and Alliance Between Selected Morphometric Features of Schilbe mystus in Asejire Reservoir, Nigeria. Asian Journal of Biological Sciences. 2023; 16(4): 555-565. https://doi.org/10.3923/ajbs.2023.555.565
Chicago/Turabian Style
Kazeem, Kareem, Oladeji, Olanrewaju Adewale Nurudeen, Oluwale Femi Victor, and Awopetu Temitope Seun.
2023. "Growth Parameters and Alliance Between Selected Morphometric Features of Schilbe mystus in Asejire Reservoir, Nigeria" Asian Journal of Biological Sciences 16, no. 4: 555-565. https://doi.org/10.3923/ajbs.2023.555.565

This work is licensed under a Creative Commons Attribution 4.0 International License.



