Effect of Pasteurization and Storage Temperatures on the Quality of Carrot Juices
| Received 16 Oct, 2023 |
Accepted 02 Dec, 2023 |
Published 31 Dec, 2023 |
Background and Objective:Unstandardized preparation methods and poor storage conditions of homemade fruit and vegetable juices could impact the quality of the product. Therefore, this study seek to determine the effect of unpasteurization and room temperature storage associated with homemade carrot juice on its microbiological, physicochemical and sensorial properties. Materials and Methods: Total heterotrophic bacterial count, total fungal count, pH, titratable acidity and total soluble solids of unpasteurized and pasteurized carrot juices stored at 0°C for 5 weeks; 4 and 24°C for 5 days were monitored at intervals using standard methods. Sensory evaluation of the samples involved the use of a 9-point hedonic scale. The data obtained were subjected to statistical analysis using a One-way Analysis of Variance (ANOVA) and probability value at p<0.05 was considered to be significant. Results: Total heterotrophic bacterial count (THBC) of pasteurized carrot juice stored at 0 and 4°C was within the range of <1.0-3.66 log10 CFU mL–1. The juices were within the THBC limits recommended by the International Commission on Microbiological Specifications of Food (ICMSF) and Codex Alimentarius Commission (CAC) of the Food and Agricultural Organization (FAO). The total fungal count of carrot juices stored at 4 and 24°C was <1.0 log10 CFU mL–1 with the exception of the unpasteurized juice stored at 24°C. During storage, the titratable acidity of the carrot juices increased from 0.3-2.8% whereas, the total soluble solids (6-23 °Brix) and pH (3.4-6.3) decreased with few exceptions. Based on sensory report, the pasteurized carrot juice stored at different temperatures was preferable than the unpasteurized juice. Conclusion: Personal and environmental hygiene, pasteurization and low temperature storage of homemade carrot juice are recommended to ensure that the products are good quality and microbiologically safe for human consumption.
| Copyright © 2023 Akpaetok et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
In recent years, the lifestyle of millions of people in terms of feeding habits has been influenced by globalization. Consequently, the number of cases of obesity, cardiovascular diseases, chronic respiratory diseases, cancer and diabetes have been on the increase. The World Health Organization (WHO) is vigorously promoting the consumption of healthy diets by recommending everyone to eat a minimum of 400 g of fruits and vegetables daily. According to statistics published by the WHO, the estimated number of deaths that occurred globally in 2013 as a result of consuming little quantity of fruits and vegetables is 6.7 million1,2. According to Pem and Jeewon3, adequate consumption of fruits and vegetables significantly reduces the risk of non-communicable diseases and helps to control obesity.
Fruits and vegetables are well-known to be rich in different types of vitamins (A, B, C and E) and minerals (Na, K, Mg, S, Ca, Mn, Cu, Fe, Zn and Cu) required by the body in small quantities to carry out metabolism and other normal functions4,5. Carrot (Daucus carota L.) is a root vegetable cultivated in all parts of the world. It is a member of the family Apiaceae6. Based on its pigmentation, carrots cultivated for human consumption are divided into two groups: Eastern carrots and Western carrots. There is no tentative information on the origin of western carrots, but eastern carrots have been traced to Afghanistan and Pakistan. According to scientists, carrots originated in both countries about 1,100 years ago7,8. There are several varieties of carrots easily identified based on colours. They include black, purple, red, yellow and white carrots9.
In terms of nutritional composition, carrots is occupy the 10th position out of 39 fruits and vegetables evaluated10,11. Carrot is popular because it is rich in β-carotene which is a precursor for vitamin A12,13. The proximate composition of carrots reported by Ahmad et al.14 shows that it contains moisture (84-95%), protein (0.6-2.0%), fat (0.2-0.7%), carbohydrates (9.58-10.6%) and fiber (0.6-2.9%). Carrots also contain vitamin C (5.9 mg/100 g), vitamin B1 (0.07 mg/100 g), vitamin B2 (0.06 mg/100 g), vitamin B3 (0.93 mg/100 g), vitamin B6 (0.138 mg/100 g), β-carotene (8285 μg/100 g), α-carotene (3477 μg/100 g) and vitamin E (0.66 mg/100 g). Minerals such as magnesium, manganese and potassium are found in reasonable amounts in carrots. Some of the minerals present in carrots help to fight tooth decay and cavities. Molybdenum is rarely found in many vegetables, however, it is present in carrots. Molybdenum is a trace mineral that helps the body to metabolize fats and carbohydrates as well as absorb iron4,13.
The presence of phytonutrients which include phenolics, polyacetylenes and carotenoids in reasonable amounts in carrots is a promoter of good health in humans15. Several health benefits associated with eating carrots regularly include fast healing of wounds, useful in fighting diabetes, cancer, hypertension, cardiac disease and reduction in the level of cholesterol in the body. Extracts from the seed possess antibacterial, antifungal, analgesic, cardio and hepatoprotective properties. Originally, the use of carrots was basically because of their medicinal properties which later became very useful as food for human consumption9,16.
In addition to chewing carrots either in raw or cooked form, it can be processed into various products which include powder, juice, candy, beverages, chips, canned carrots, kheer and halwa. Preparation of soups, beverages, curries, wine, jams, stews and pies could be enhanced by incorporating carrots5,14. Consumption of carrot juice helps to flush the kidney. It is also helpful in relieving congestion or inflammation of the kidney. Daily consumption of a cup of carrot juice helps to improve eyesight, skin, blood sugar level and healthiness of the blood. Since β-carotene is a precursor of vitamin A, carrot juice helps to protect the eyes and skin; fight against free radicals which cause damage in the body leading to the development of chronic diseases (heart disease and cancer); helps boost the functions of the brain, immune system and cognitive function. It also helps in reducing the risk of dementia and conditions associated with memory loss. Carrot juice is helpful for lactating mothers because it enriches breast milk with nutrients for the baby1,13.
A lot of people enjoy drinking unpasteurized vegetable and fruit juice because it is fresh, rich in vitamins and the calorie content is low17. Due to increasing awareness about healthy lifestyles and diets, an increasing number of consumers of fruit and vegetable juice prefer the product prepared at home without adding preservatives to commercially packed juices that contain chemical preservatives. Without pasteurization, ready-to-drink vegetable and fruit juice is likely to contain a high microbial load including potentially harmful microorganisms. Therefore, the product will have a short shelf life. A study carried out by Adeleke et al.18 reported that carrots sold in some markets were contaminated with Bacillus cereus, Staphylococcus aureus, Enterococcus faecalis, Citrobacter sp. and Candida sp. The consumption of unpasteurized carrot juice prepared using carrots bought from the market could lead to food-borne illnesses. Most people’s immune systems can fight off the symptoms of certain food-borne illnesses. However, children, the elderly and immunocompromised individuals are at risk of becoming ill as a result of drinking unpasteurized carrot juice. Therefore, this study is aimed at determining the effect of pasteurization on microbiological, physicochemical and sensorial properties of carrot juice stored at 0°C, 4°C and ambient temperature (24°C).
MATERIALS AND METHODS
Study area: The study was carried out in the Microbiology Laboratory, University of Port Harcourt, Choba, Rivers State. The duration of the study is 6 weeks; between May to June, 2023.
Preparation of carrot juice: About 14 kg of Nantes variety of carrots (Daucus carota sp., sativa) were bought from a local vegetable fruit garden market in D-line, Port Harcourt using a sterile polyethene bag. Before processing the carrots, they were washed with potable water. Thereafter, the head and bottom of each carrot were cut off followed by peeling the bark with a clean stainless steel knife sterilized with 70% ethanol. A repeat washing of the peeled carrots was done. The clean peeled carrots were diced (20×20×20 mm) and soaked in potable water for 30 min. The diced carrot (12 kg) was macerated with 12 L of potable water and the carrot juice was extracted using a juice extractor (Kenwood Accent Collection Juicer, JEM02). The carrot juice was poured inside a sterile universal sample container (30 mL). A total of 90 universal sample containers were filled with carrot juice and tightly corked. Half of the total number of containers containing carrot juice (45 samples) were pasteurized using the low-temperature long time method (63°C for 30 min) whereas the remaining half of the containers (45 samples) were unpasteurized.
Storage of carrot juice: Immediately after pasteurization was completed, both pasteurized and unpasteurized carrot juice samples were stored at 0, 4 and 24°C (ambient temperature). Samples of unpasteurized and pasteurized samples stored at 24°C and 4°C were withdrawn daily for microbiological and physicochemical analysis. Similarly, samples from the unpasteurized and pasteurized samples stored at 0°C were withdrawn at 1 week interval for microbiological analysis.
Serial dilution: A 10-fold serial dilution for each sample of carrot juice was carried out using sterile buffered peptone water. Exactly 10 mL of carrot juice sample was diluted with 90 mL of 0.1 % sterile peptone water. Serial dilution upto 10–5 was done by transferring 1 mL of the stock solution into subsequent test tubes containing 9 mL sterile peptone water using a sterile pipette for each transfer.
Determination of total aerobic plate count: Dilutions of 10–3 and 10–5 for each sample of carrot juice was surface spread onto duplicate sterile nutrient agar (NA) plates for enumeration of bacteria. The inoculated plates were incubated at 37°C for 24 hrs. Uninoculated NA plate served as the control. After incubation, discreet colonies on NA plates were manually counted and recorded. The formula below described by Ahaotu et al.19 was used to calculate the aerobic plate count expressed as Colony Forming Units per mL (CFU mL–1):
Determination of total fungal count: Exactly 1 mL solution from dilutions 10–3 and 10–5 were surface spread onto duplicate potato dextrose agar (PDA) plates supplemented with antibiotics for enumeration of fungi. The inoculated agar plates were incubated at 28±2°C for 5 days. Uninoculated PDA plate served as the control. After incubation, discreet colonies on PDA plates were manually counted and recorded. The fomula below was use to calculate the total fungal count expressed as Colony Forming Units per mL (CFU mL–1):
 |
Obtaining pure cultures: Distinct colonies were randomly collected using a sterile wire loop and sub-cultured on freshly prepared sterile NA plates and incubated for 24 hrs at 37°C for bacterial isolates. A similar procedure was carried out for fungal isolates which were sub-cultured on freshly prepared sterile PDA plates and incubated at room temperature (28±2°C) for 5 days to obtain pure cultures.
Identification of bacterial isolates: Bacterial isolates were identified based on microscopic, colonial morphology and biochemical characteristics with reference to Bergey and Holt20 and Shoaib et al.21. Gram staining and motility test of the bacterial isolates was performed. Biochemical tests of each bacterial isolates involved catalase test, oxidase test, citrate test, indole test, sulphur test, sugar fermentation, triple sugar iron test and Methyl-Red-Voges-Proskauer test21,22.
Identification of fungi isolates: Pure fungal isolates were placed on clean and grease free slide and a drop of lactophenol was added. The preparation was covered with coverslip. Immediately, the preparation was observed under ×10 and ×40 objective lenses. Identification of the fungi isolates was done by comparison of observed features with a fungi atlas23.
Physicochemical analysis
pH: The pH of carrot juice sample was measured at room temperature (28±2°C) using a digital pH meter (Thermo Orion, 710A+). A standard buffer solution prepared with 1.23 g of boric acid (0.2 N) and 1.49 g of potassium chloride in 100 mL of distilled water was used as solution for standard pH. Carrot juice was poured inside a graduated cylinder until it reached 30 mL mark. The probe of the pH meter was dipped inside the carrot juice sample for 3 min. When the figure on the display was stable, the pH reading was taken. The measurement was carried out in duplicate for each sample.
Total soluble solids (TSS): The method described by Kong et al.24 was adopted with slight modification. The TSS were determined as °Brix at 20±1°C using a refractometer (Atago Master T, Tokyo, Japan). The device was lowered in each sample of carrot juice and reading was taken within 3 min. The test was performed in duplicate for each sample.
Titratable acidity: Titratable acidity of each sample of carrot juice was determined by neutralizing 100 cm3 of the carrot juice with 0.1 M of Sodium Hydroxide (NaOH) using phenolphthalein as indicator. The result was expressed as percentage of malic acid. The test was performed in duplicate for each sample.
Sensory evaluation: A total of 10 semi-trained panelists carried out a preference ranking test to assess the taste, aroma, mouthfeel, appearance, colour and overall acceptability of pasteurized and unpasteurized carrot juices stored at different temperatures. Ranking of the carrot juice in terms of colour was carried out by the panelist inside a cabinet where standard light was available. Carrot juice inside glass bottles were presented to the panelist in a similar manner commercialized juice is served. The preference ranking test was used by the panelist to assess the colour of the juice in a cabinet where there was a standardized light. Twenty five milliliter of each sample of carrot juice was poured inside a labeled plastic glasses and seved each panelist. The flavour of the samples were assessed independently by the panelist. Order of presentation of the carrot juices was based on a balanced-block design. Assessment of sensory attributes of each carrot juice involved the use of nine-point hedonic scale which range from 1 (dislike extremely) to 9 (like extremely)25.
Statistical analysis: Data obtained in replicates were subjected to One-way Analysis of Variance (ANOVA) with the help of IBM SPSS Statistics version 23 software. A probability value at p<0.05 was considered to be statistically significant. Duncan’s Multiple Range Test (DMRT) was used to separate the means.
RESULTS
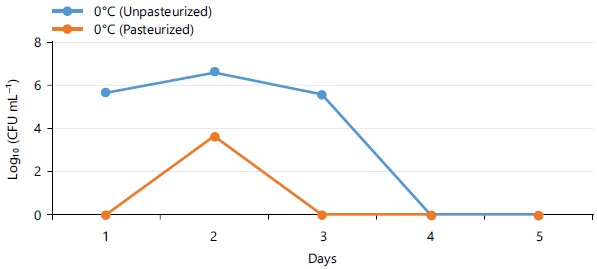
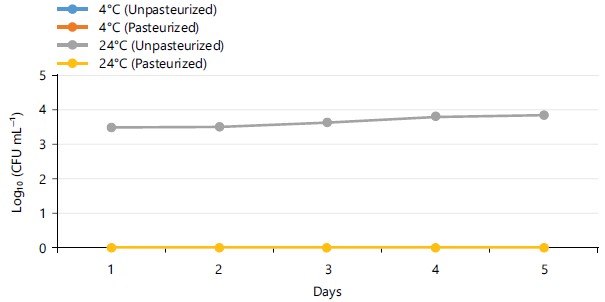
The total heterotrophic bacterial count (THBC) of pasteurized and unpasteurized carrot juice samples stored at different temperatures (4 and 24°C) for 5 days was shown in Fig. 1. The THBC of unpasteurized carrot juice stored at 4°C and 24°C was within the range of 6.301 to 6.89 and 7.31 to 9.32 log10 CFU mL–1, respectively. As for the pasteurized carrot juice stored at 4°C, the THBC was <1.0 log10 CFU mL–1 while the result for the samples stored at 24°C was within the range of 4.47 to 6.69 log10 CFU mL–1. Figure 2 shows the THBC of pasteurized and unpasteurized carrot juice stored at 0°C for 5 weeks. At 0°C, the highest THBC recorded for unpasteurized and pasteurized carrot juice was 6.63 and 3.66 log10 CFU mL–1, respectively.

|
|
|
| Table 1: | Gram stain, morphology and biochemical characteristics of bacterial isolates | |||
Isolate code |
Gram stain |
Morphology |
Catalase |
Oxidase |
Sucrose |
Glucose |
Lactose |
Starch |
TSIA slant |
Agar butt |
Gas |
H2S |
Indole |
MR |
VP |
Citrate |
Motility |
Probable genera |
A |
+ |
R |
+ |
- |
+ |
+ |
- |
- |
B |
A |
- |
+ |
- |
+ |
- |
+ |
+ |
Bacillus sp. |
B |
+ |
C |
+ |
- |
+ |
+ |
+ |
+ |
B |
A |
- |
- |
- |
+ |
+ |
+ |
- |
Staphylococcus sp. |
C |
- |
R |
+ |
+ |
+ |
+ |
- |
+ |
B |
B |
- |
- |
- |
+ |
- |
+ |
+ |
Pseudomonas sp. |
D |
- |
R |
+ |
- |
+ |
+ |
- |
+ |
B |
A |
+ |
- |
- |
- |
+ |
- |
+ |
Serratia sp. |
| TSIA: Triple sugar iron agar, MR: Methyl red, VP: Voges proskauer, R: Rod, C: Cocci, A: Acid, B: Base, +: Positive and -: Negative | ||||||||||||||||||
Figure 3 shows the total fungal count (TFC) of pasteurized and unpasteurized carrot juice stored at different temperatures (4 and 24°C) for 5 days. The TFC of unpasteurized carrot juice slightly increased from 3.5 to 3.85 log10 CFU mL–1 during the period of storage at 24°C whereas the result for pasteurized sample was <1.0 log10 CFU mL–1. Within the same period of storage, the TFC of both pasteurized and unpasteurized carrot juice stored at 4°C was <1.0 log10 CFU mL–1. A similar result was recorded for both pasteurized and unpasteurized carrot juice stored for 5 weeks at 0°C.
Table 1 shows the result of biochemical tests and Gram staining of bacterial isolates from both pasteurized and unpasteurized carrot juice. The result indicates that 4 bacterial genera which include Bacillus sp., Staphylococcus sp., Pseudomonas sp. and Serratia sp. were encountered in the carrot juice samples.
Bacillus sp. (43 %) which accounted for the highest frequency of occurrence was found in all the samples of unpasteurized and pasteurized carrot juice stored at 0, 4 and 24°C. It is followed by Serratia sp. (33%) reported in all carrot juice samples stored at different temperatures (0, 4 and 24°C). Pseudomonas sp. (16%) was isolated from unpasteurized carrot juice stored at 24°C and 4°C. The bacterial isolate which had the lowest frequency of occurrence was Staphylococcus sp. (8%) isolated from unpasteurized carrot juice stored at 24°C.
The fungi genera which had the highest frequency of occurrence was Penicillium spp. (45%) isolated from pasteurized and unpasteurized carrot juice stored at 24°C and 4°C for 5 days. It is followed by Saccharomyces spp. (37%) which was encountered in both pasteurized and unpasteurized carrot juice stored at different temperatures (4 and 24°C). Aspergillus spp. (18%) isolated from unpasteurized carrot juice stored at 24°C is the fungal genera that had the lowest frequency of occurrence.

|

|
Figure 4 shows the pH of unpasteurized and pasteurized carrot juice stored at different temperatures (4 and 24°C) for 5 days. At 4°C, the pH of unpasteurized and pasteurized carrot drink was within the range of 5.3-5.6 and 6.1-6.3, respectively. When the storage temperature was increased to 24°C, the pH of unpasteurized and pasteurized carrot drink was within the range of 3.4-4.4 and 4.4-4.9, respectively. The pH of unpasteurized (3.2-6.3) and pasteurized (4.1-6.3) carrot juice stored for 5 weeks at 0°C was presented in Fig. 5.
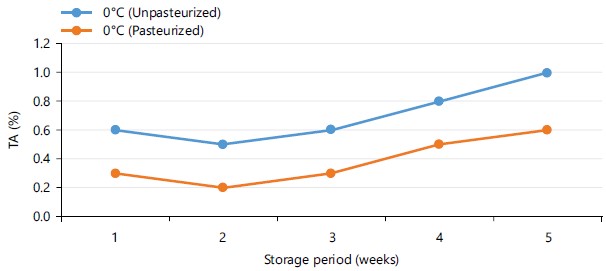
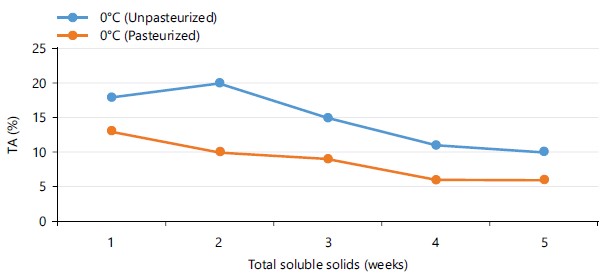
Figure 6 shows the titratable acidity (TA) of pasteurized and unpasterurized carrot juice stored at 4 and 24°C for 5 days. The results shows there was a steady increase in TA of both samples with the exception of pasteurized carrot juice stored at 0°C which slightly reduced from 0.7 to 0.3% within 3 days of storage, but increased in subsequent days. Figure 7 shows the TA of pasteurized and unpasteurized carrot juice stored at 0°C for 5 weeks. Within 2 weeks of storage, there was a decrease in TA of both pasteurized and non-pasteruized carrot juice which later increased steadily in subsequent weeks.
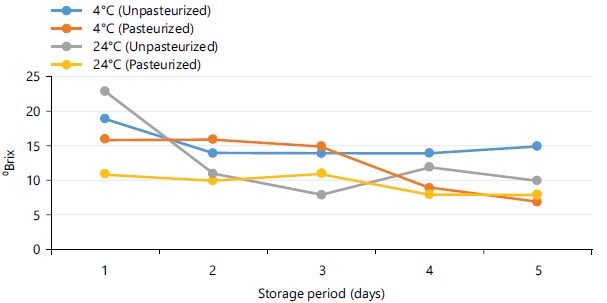
Figure 8 shows the total soluble solids of pasteurized and unpasteurized carrot juice stored at different temperatures. During the period of storage, reduction in TSS in all the samples of carrot juice occurred except at few intervals when the values were the same or slightly increased. The TSS of unpasteurized carrot juice stored at 4 and 24°C was within the range of 14-19 and 8-23 °Brix. The TSS within the range of 7-16 and 8-11 °Brix was reported in pasteurized samples of carrot juice stored at 4 and 24°C, respectively for 5 days.

|
|
|
|
| Table 2: | Sensory evaluation of pasteurized and unpasteurized carrot juices at day 1 | |||
CAAP |
CABP |
|||||
| Parameter | CAAP1 |
CAAP2 |
CAAP3 |
CABP1 |
CABP2 |
CABP3 |
| Taste | 6.1±1.29b |
6.3±1.16b |
6.0±1.05b |
4.1±0.74a |
4.3±0.67a |
4.1±0.88a |
| Aroma | 6.2±0.63b |
5.4±1.07b |
5.4±1.58b |
3.4±0.70a |
3.1±1.10a |
4.0±1.49a |
| Mouth feel | 7.3±0.95d |
6.3±0.95c |
6.2±1.03c |
4.0±0.82a |
4.2±0.92a |
5.3±0.95b |
| Appearance | 8.0±1.05b |
7.2±0.92ab |
8.0±1.25b |
7.1±1.29ab |
8.1±1.10b |
6.2±1.23a |
| Colour | 8.1±0.88ab |
8.1±1.10ab |
7.1±1.20a |
9.0±0.00b |
8.0±1.25a |
7.2±1.14a |
| Overall acceptability | 7.1±0.99c |
7.1±1.10c |
7.3±0.95c |
5.4±0.84b |
4.4±0.84a |
5.0±1.05ab |
Values show means of sensory scores of ten panelists ±SD, Values with different superscript across the row are significantly different (p<0.05), Hedonic scale: 9: Like extremely, 8: Like very much, 7: Like moderately, 6: Like slightly, 5: Neither liked nor disliked, 4: Disliked slightly, 3: Disliked moderately, 2: Disliked very much, 1: Disliked extremely, CAAP1: Pasteurized carrot juice stored at 0°C, CAAP2: Pasteurized carrot juice stored at 4°C, CAAP3: Pasteurized carrot juice stored at 24°C, CABP1: Unpasteurized carrot juice stored at 0°C, CABP2: Unpasteurized carrot juice stored at 4°C, CABP3: Unpasteurized carrot juice stored at 24°C |
||||||
| Table 3: | Sensory evaluation of pasteurized and unpasteurized carrot juices at day 5 | |||
CAAP |
CABP |
|||||
| Parameter | CAAP1 |
CAAP2 |
CAAP3 |
CABP1 |
CABP2 |
CABP3 |
| Taste | 5.2±1.03a |
6.2±1.03a |
6.3±1.25a |
5.2±1.23a |
5.4±0.97a |
5.2±1.03a |
| Aroma | 5.1±0.99a |
5.4±1.17a |
5.3±0.82a |
5.0±0.94a |
5.4±0.84a |
5.1±0.99a |
| Mouth feel | 4.4±0.84ab |
4±0.82a |
5.3±0.95b |
5.1±1.20ab |
5.0±0.82ab |
5.1±1.20ab |
| Appearance | 6.1±0.99abc |
6.4±1.17bc |
6.0±1.25ab |
5.2±1.03a |
7.2±0.99c |
7.1±0.74c |
| Colour | 6.4±1.17ab |
7.2±1.03b |
7.0±0.94ab |
6.0±1.05a |
7.0±1.15ab |
7.0±0.94ab |
| Overall acceptability | 6.4±1.07b |
6.3±0.88ab |
6.3±1.34b |
5.1±0.99a |
6.0±1.05ab |
6.3±1.06b |
Values show means of sensory scores of ten panelists ±SD, Values with different superscript across the row are significantly different (p<0.05), hedonic scale: 9: Like extremely, 8: Like very much, 7: Like moderately, 6: Like slightly, 5: Neither liked nor disliked, 4: Disliked slightly, 3: Disliked moderately, 2: Disliked very much, 1: Disliked extremely, CAAP1: Pasteurized carrot juice stored at 0°C, CAAP2: Pasteurized carrot juice stored at 4°C, CAAP3: Pasteurized carrot juice stored at 24°C, CABP1: Unpasteurized carrot juice stored at 0°C, CABP2: Unpasteurized carrot juice stored at 4°C and CABP3: Unpasteurized carrot juice stored at 24°C |
||||||
Figure 9 shows the total soluble solids (TSS) of pasteurized and unpasteurized carrot juices stored at 0°C for 5 weeks. In both samples of carrot juice, there was a steady reduction in TSS during the period of storage with the exception of unpasteurized carrot juice which slightly increased at week 2. The TSS of unpasteurized and pasteurized carrot juice stored at 0°C was within the range of 10-20 and 6-13 °Brix, respectively.
The sensory evaluation report of pasteruirzed and unpasterurized carrot juice at day 1 is presented in Table 2. According to the report, pasteurized carrot juice stored at 0, 4 and 24°C had a higher sensory scores compared to unpasteurized juices stored under the same condition. Table 3 shows the sensory evaluation report of pasteurized and unpasteurized carrot juices stored at different temperatures at day 5. The report shows that pasteurized carrot juices had a slightly higher scores for all the sensory attributes compared to unpasteurized carrot juices with few except.
DISCUSSION
The result obtained from this study shows that the total heterotrophic bacterial count (THBC) of unpasteurized carrot juice was higher than that pasteurized juice. It could be attributed to high temperature involved in pasteurization which killed a lot of microbes present in carrot juice. This report was in agreement with the findings of Jimma et al.26 In addition, low temperature storage could also have contributed to reducing the microbial load of the carrot juices. Kaddumukasa et al.27, reported a similar trend in a related study. According to Oranusi et al.28, edible products containing microbial contaminants below 6 log10 CFU mL–1 are acceptable for humans to consume. Meanwhile, the International Commission on Microbiological Specifications of Food (ICMSF) and the National Agency for Food and Drug Administration and Control (NAFDAC) stipulate that mesophilic aerobic bacterial count in fruit juice should not be above 3 log10 CFU mL–1 29. Codex Alimentarius Commission (CAC) of the Food and Agricultural Organization (FAO) recommend that the total mean plate count of fruit juice should not exceed 1×105 CFU mL–1 27. Pasteurized carrot juice stored at 4°C for 5 days met the ICMSF and NAFDAC standards. A similar result also involved carrot juices stored at 0°C for 5 weeks except that THBC of the pasteurized juice exceeded the recommended limit at week 2; the unpasteurized carrot juice at weeks 1, 2 and 3. Pasteurized and unpasteurized carrot juices stored at 24°C for 5 days exceeded the permissible limit for mesophilic aerobic bacterial count recommended by the CAC, ICMSF and NAFDAC.
Asghar et al.30 reported that total plate count, Staphylococcus aureus, yeast and mould count of fresh carrot juice sold by vendors is within the range of 4.5×103-5.5×105, <10-6.0×103 and 6.7×101-8.5×103 per millilitre of the juice, respectively. Total coliforms, fecal coliform and Escherichia coli were not detected in some samples of the juice, but the highest values encountered in others were 460, 240 and 93 MPN mL–1, respectively. Salmonella sp., was detected in 3 out of 10 samples of the carrot juice. In a related study, Asha et al.31 reported that the TVC and coliform count of carrot juice sold to the public is within the range of 80-271×105 CFU mL–1 and 1-54×105 CFU mL–1, respectively. Both reports are in agreement with the results obtained from this study. Ghosh et al.32 reported that total aerobic count, total staphylococcal count, total coliforms, total fecal coliforms of carrots obtained from street vendors before washing the root vegetable is within the range of 5.0-798, 4.81-7.56, 2.0-5.97 and 3.2-8.2 log10 CFU mL–1 while the values reported for washed carrots is 3.1-6.4, 1.25-5.0, 2.08-6.67 and 5.80-8.33 log10 CFU g–1, respectively. Fruits and vegetables are easily contaminated by microorganisms when they are allowed to fall on the ground during harvesting. The use of dirty water to wash fruits and vegetables could contaminate them with fecal pathogens. Other possible sources of microbial contamination of carrot juice include dust, air and personnel during the preparation of the juice27.
Our results showed that unpasteruized carrot juice stored at 24°C for 5 days had a total fungal count within the range of 3.5-3.85 log10 CFU mL–1. Other samples of carrot juice stored at 4°C and 24°C were <1.0 log10 CFU mL–1. The effect of pasteruization and low-temperature storage could be responsible for the low TFC of the carrot juices. Poor storage conditions and improper handling of carrots beginning from the stage of harvesting to processing of carrots into juice are possible sources of fungal contamination of the ready-to-drink juice33. Asha et al.31 reported that the yeast count of street vended carrot juice is within the range of 5-41×105 CFU mL–1.
The dominance of Bacillus sp. in the carrot juice samples stored at different temperatures could be attributed to the bacterium being ubiquitous in nature and a spore former19. The presence of bacterial species in the carrot juice is an indication they can survive in an acidic environment. This is probably because the organisms are capable of regulating their internal pH which they adjusted to neutral by deploying two mechanisms-passive and active homeostasis34. According to Oranusi et al.28, bacterial species encountered in an edible product shows the quality of raw materials, the hygienic status of the environment, process equipment, packaging materials and personnel handling the product.
Serratia marscescens was reported in carrots sold in selected markets in Ekiti State18. Serratia sp., is a bacterium whose presence in processing plants, water, soil, insects and vertebrates has been reported. Aneja et al.34 reported that Serratia sp., contaminated freshly prepared carrot juice. This is in agreement with the findings from this study. According to Adeleke et al.18, Serratia marscescens is associated with meningitis in children.
With the exception of Pseudomonas sp., all the bacterial genera encountered in carrot juices were reported in a related study by Aneja et al.34. Pseudomonas sp., is a common food contaminant associated with spoilage. Morka35 reported that Pseudomonas aeruginosa is among the bacteria implicated in the spoilage of carrots sold in the market. According to Igiehon et al.36 Pseudomonas sp., isolated from vended fruits could cause necrotizing inflammation in the body.
Staphylococcus sp., isolated from the carrot juice could be the specie that usually inhabit the skin, hair, palm, nose and mucus membrane as part of the normal flora. The bacterium is commonly associated with post-process contamination of food products such as carrot juice28. A possible source of contamination of carrot juice by Staphylococcus sp., is the food handlers. According to Ire et al.37 carbuncles, furuncles, folliculitis, erysipelas, scalded skin syndrome (toxemia) and cellulitis are human diseases associated with Staphylococcus aureus.
According to Oranusi et al.28 spoilage of fruits and vegetables is usually caused by fungi such as Aspergillus and Penicillium species. Aneja et al.34 reported that freshly prepared carrot juice was contaminated by Saccharomyces sp., Aspergillus flavus, A. terreus, A.niger and Penicillium islandicum. The study further revealed that Aspergillus flavus and Saccharomyces sp., were among the dominant mould and yeast encountered in the carrot juice, respectively. The result is reasonably in agreement with the findings from this study.
There was a slight reduction in the pH of unpasteurized carrot juice stored at 24°C for 5 days from 4.4-3.4. This could be attributed to favourable temperature for fermentative bacteria in the carrot juice to carry out their activities. Secondly, an increase in titratable acidity in the samples could also be a contributory factor because of the inversely proportional relationship between pH and titratable acidity. Hashem et al.38 reported that pH of freshly prepared carrot juice is 6.35±0.16. The standard for pH of fruit juice recommended by FAO/WHO and Standard Organization of Nigeria (SON) is 1.4-4.039.
The titratable acidity (TA) of pasteurized and unpasteurized carrot juice stored at different temperatures increased during the period of storage. Increase in acidity of pasteurized carrot juice stored at room temperature from 0.05±0.01-0.08±0.01% reported by Kapoor and Aggarwal40 is in agreement with the trend reported in this study. Hashem et al.38 reported that acidity of freshly prepared carrot juice is 0.38±0.17%. The increase in TA of carrot juice during the period of storage could be attributed to organic acid undergoing partial hydrolysis41.
The result from this study showed that during storage of carrot juice at different temperatures, there was a reduction in total soluble solids (TSS). It could be attributed to the activities of microorganisms that break down sugar in the juices and become acids42. In a related study, Kapoor and Aggarwal40 reported that TSS of tightly corked and pasteurized carrot juice stored at room temperature for 6 months reduced from 10±0.03-9.73±0.11 °Brix. This is in agreement with our results. Hashem et al.38 reported that the total soluble solids in freshly prepared carrot juice are 4.17±0.01 °Brix.
The sensory evaluation report of freshly prepared carrot juices on day 1 showed that there was no significant difference (p>0.05) in taste and aroma among the pasteurized and unpasteurized samples stored at 0, 4 and 24°C. Similarly, there was no significant difference (p>0.05) in overall acceptability among the pasteurized samples which contradicted the report of unpasteurized samples. This result could be attributed to pasteurization of carrot juice which significantly reduced the population of microorganisms capable of releasing metabolites into the juice that will influence its overall acceptability. The sensory report of day 1 indicates that pasteurized carrot juice stored at 0, 4 and 24°C was preferable to the samples which were unpasteurized. On day 5, the taste and aroma among the carrot juice samples were not significantly different (p>0.05). It is worth noting that sensory scores for mouthfeel, appearance, colour and overall acceptability of pasteurized and unpasteurized carrot juice samples on day 1 were higher than what was reported in the samples on day 5. This could be attributed to the activities of microorganisms in the products which increased in population during the period of storage. Consequently, the activities of the microorganisms probably were responsible for breaking down sugar in carrot juice and releasing metabolites which impacted the sensory properties of the product.
CONCLUSION
This study has shown that unpasteurization of carrot juice and storage of the product at room temperature which are common practices in many households make the product unfit for human consumption. However, pasteurization and storage of carrot juices at low temperatures improved the microbiological and sensorial quality of the product. Further studies on the effect of pasteurization and storage temperatures on the proximate composition and mineral content of homemade carrot juice is recommended.
SIGNIFICANCE STATEMENT
Increasing concern about the health effects of artificial flavours and preservatives in many brands of fruit and vegetable juices has made homemade juice to be a good alternative. Unpasteurization and room temperature storage associated with homemade juice could affect its quality and pose a health risk to consumers. The effect of both conditions on the microbiological, physicochemical and sensory parameters of carrot juice was determined. A bacterial load of pasteurized carrot juice stored at 4°C for 5 days; at 0°C for 5 weeks was within the recommended limits while changes in pH, titratable acidity and total soluble solids occurred. The pasteurized carrot juice stored at different temperatures had better sensory attributes compared with the unpasteurized juices.
ACKNOWLEDGMENTS
The authors hereby appreciate the support given to us by the Chief Laboratory Technologist and other support staff at General Microbiology Laboratory, Universisty of Port Harcourt, during the laboratory work.
REFERENCES
- Potter, A.S., S. Foroudi, A. Stamatikos, B.S. Patil and F. Deyhim, 2011. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr. J., 10.
- Raggio, L. and A. Gámbaro, 2018. Study of the reasons for the consumption of each type of vegetable within a population of school-aged children. BMC Public Health, 18.
- Pem, D. and R. Jeewon, 2015. Fruit and vegetable intake: Benefits and progress of nutrition education interventions- Narrative review article. Iran. J. Public Health, 44: 1309-1321.
- Ajenu, C.O., C. Imoisi, E.E. Imhontu and U.R.Orji, 2021. Comparative evaluation of the proximate and micro-nutritional benefits of pawpaw, carrot, turmeric and coconut. J. Food Technol. Nutr. Sci., 3: 1-5.
- Yahia, E.M., P. García-Solís and M.E.M. Celis, 2019. Contribution of Fruits and Vegetables to Human Nutrition and Health. In: Postharvest Physiology and Biochemistry of Fruits and Vegetables, Yahia, E.M. (Ed.), Woodhead Publishing, Sawston, Cambridge, ISBN: 9780128132784, pp: 19-45.
- Surbhi, S., R.C. Verma, R. Deepak, H.K. Jain and K.K. Yadav, 2018. A review: Food, chemical composition and utilization of carrot (Daucus carota L.) pomace. Int. J. Chem. Stud., 6: 2921-2926.
- Que, F., X.L. Hou, G.L. Wang, Z.S. Xu and G.F. Tan et al., 2019. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res., 6.
- Boadi, N.O., M. Badu, N.K. Kortei, S.A. Saah and B. Annor et al., 2021. Nutritional composition and antioxidant properties of three varieties of carrot (Daucus carota). Sci. Afr., 12.
- Varshney, K. and K. Mishra, 2022. An analysis of health benefits of carrot. Int. J. Innovative Res. Eng. Manage., 9: 211-214.
- da Silva Dias, J.C., 2014. Nutritional and health benefits of carrots and their seed extracts. Food Nutr. Sci., 5: 2147-2156.
- Bratte, A.G., A.S. Adeleye and A.S. Oyerinde, 2016. Comparison of nutritional and colour properties of fresh and dried carrot (Daucus carota L.) slices and carrot pomace. J. Multidiscip. Eng. Sci. Technol., 3: 5366-5370.
- Aderinola, T.A. and K.E. Abaire, 2019. Quality acceptability, nutritional composition and antioxidant properties of carrot-cucumber juice. Beverages, 5.
- Prasad, S., 2021. Development, analysis and sensory evaluation of carrot squash as a natural health drink. Int. J. Innovative Res. Technol., 8: 1071-1077.
- Ahmad, T., M. Cawood, Q. Iqbal, A. Ariño and A. Batool et al., 2019. Phytochemicals in Daucus carota and their health benefits-review article. Foods, 8.
- Hager, T.J. and L.R. Howard, 2006. Processing effects on carrot phytonutrients. Am. Soc. Hortic. Sci., 41: 74-79.
- Singh, M.N., R. Srivastava and I. Yadav, 2021. Study of different varietis of carrot and its benefits for human health: A review. J. Pharmacogn. Phytochem., 10: 1293-1299.
- Hammad, A.A., H.H. Abd El-Khalek, K.A. Youssef and R.M. Abd El-Kader, 2013. Microbiological, nutritional and sensorial changes in fresh carrot juice preserved by irradiation. Food Sci. Qual. Manage., 11: 61-69.
- Adeleke, M.A., A.O. Hassan, T.T. Ayepola, T.M. Famodimu, W.O. Adebimpe and G.O. Olatunde, 2012. Public health risks associated with apples and carrots sold in major markets in Osogbo, Southwest Nigeria. J. Toxicol. Environ. Health Sci., 4: 140-144.
- Ahaotu, I., M.O. Ichendu and N. Maduka, 2022. Microbiological, nutritional and sensory evaluation of snack bars developed using Bambara groundnut (Vigna subterranean L.) and maize (Zea mays). Afr. J. Microbiol. Res., 16: 8-23.
- Bergey, D.H. and J.G. Holt, 1994. Bergey's Manual of Determinative Bacteriology. 9th Edn., Williams and Wilkins, Baltimore, Maryland, ISBN: 978068300603, Pages: 787.
- Shoaib, M., I. Muzammil, M. Hammad, Z.A. Bhutta and I. Yaseen, 2020. A mini-review on commonly used biochemical tests for identification of bacteria. Int. J. Res. Publ., 54.
- Palma, V., M.S. Gutiérrez, O. Vargas, R. Parthasarathy and P. Navarrete, 2022. Methods to evaluate bacterial motility and its role in bacterial-host interactions. Microorganisms, 10.
- Kidd, S., C.L. Halliday, H. Alexiou and D.H. Ellis, 2016. Descriptions of Medical Fungi. 3rd Edn., CutCut Digital, New York, ISBN-13: 9780646951294, Pages: 264.
- Kong, T.Y., N.Z.N. Hasnan, A.N. Diyana, I.M. Nurin-Zulkifli, R.K. Basha, N.H. Abdul Ghani and N.A. Aziz, 2020. Effect of mild-temperature and high-temperature pasteurisation on physicochemical properties, bioactive compound, antioxidant activity and microbiological qualities of reconstituted pomegranate juice (RPJ). Food Res., 4: 157-164.
- Tiencheu, B., D.N. Nji, A.U. Achidi, A.C. Egbe and N. Tenyang et al., 2021. Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), Honey and Ginger (Zingiber officinale). Heliyon, 7.
- Jimma, F.I., A. Mohammed, E.G. Adzaworlu, J. Nzeh, L. Quansah and O.A. Dufailu, 2022. Microbial quality and antimicrobial residue of local and industrial processed fruit juice sold in Tamale, Ghana. Discover Food, 2.
- Kaddumukasa, P.P., S.M. Imathiu, J.M. Mathara and J.L. Nakavuma, 2017. Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Sci. Nutr., 5: 1098-1105.
- Oranusi, S.U., W. Braide and H.O. Nezianya, 2012. Microbiological and chemical quality assessment of some commercially packed fruit juices sold in Nigeria. Greener J. Biol. Sci., 2: 1-6.
- Obasi, B.C., C.M.Z. Whong, J.B. Ameh and E.E. Ella, 2019. Microbiological quality assessment of commercially and laboratory prepared orange juice. J. Biotechnol. Res., 5: 19-27.
- Asghar, U., M. Nadeem, R. Nelofer, S. Mazhar, Q. Syed and M. Irfan, 2018. Microbiological assessment of fresh juices vended in different areas of Lahore City. Electron. J. Biol., 14: 106-110.
- Asha, S., K. Nithisha, G. Niteesha, K.R. Bharath and V. Ravikumar, 2014. Evaluation of microbial quality of street vended vegetable and fruit juices. Int. Res. J. Biol. Sci., 3: 60-64.
- Ghosh, M., A. Ganguli and S. Mudgil, 2004. Microbiological quality of carrots used for preparation of fresh squeezed street vended carrot juices in India. J. Food Agric. Environ., 2: 143-145.
- Batool, S.A., S.S. Tahir, N. Rauf and R. Kalsoom, 2013. Microbiological analysis of pasteurized and fresh fruit juice sold in Rawalpindi of Pakistan. Bangladesh J. Sci. Ind. Res., 48: 185-192.
- Aneja, K.R., R. Dhiman, N. K. Aggarwal, V. Kumar and M. Kaur, 2014. Microbes associated with freshly prepared juices of citrus and carrots. Int. J. Food Sci., 2014.
- Morka, E., 2022. Isolation of some pathogenic microbes associated with spoilt carrots (Daucus carota L.) obtained from local markets in Abraka, Delta State, Nigeria. Dutse J. Pure Appl. Sci., 8: 1-8.
- Igiehon O.O., A.E. Adekoya and A.T. Idowu, 2020. A review on the consumption of vended fruits: microbial assessment, risk, and its control. Food Quality and Safety 4: 77-81.
- Ire, F.S., G.K. Benneth and N. Maduka, 2020. Microbiological evaluation of ready-to-drink tigernut drinks sold within port harcourt metropolis, rivers state, Nigeria. Asian Food Sci. J., 16: 45-58.
- Hashem, H.A., A.M. Sharaf, S.A. Amira and G.E. Ibrahim, 2014. Changes in physico-chemical quality and volatile compounds of orange-carrot juice blends during storage. Food Sci. Qual. Manage., 33: 21-35.
- Okoye, C.O.B. and C.N. Ibeto, 2009. Analysis of different brands of fruit juice with emphasis on their sugar and trace metal content. Bio-Research, 7: 493-495.
- Kapoor, S. and P. Aggarwal, 2014. Effect of processing and storage on bioactive compounds and antioxidant activity of carrot juice. J. Appl. Hortic., 16: 80-84.
- Babarinde, G.O., S.J. Olatunde and A. Adebiyi-Olabode, 2019. Quality attributes and phytochemical properties of fresh juice produced from selected mango varieties. Ceylon J. Sci., 48: 31-36.
- Mathew, B., V. Mini, J.M. Kuriakose, V.R. Shajan and G. Jayakumar, 2016. Effect of thermo chemical processing on storability of sugarcane juice. Int. J. Environ. Agric. Biotechnol., 1: 526-530.
How to Cite this paper?
APA-7 Style
Akpaetok,
D.E., Ahaotu,
I., Maduka,
N. (2023). Effect of Pasteurization and Storage Temperatures on the Quality of Carrot Juices. Asian Journal of Biological Sciences, 16(4), 600-613. https://doi.org/10.3923/ajbs.2023.600.613
ACS Style
Akpaetok,
D.E.; Ahaotu,
I.; Maduka,
N. Effect of Pasteurization and Storage Temperatures on the Quality of Carrot Juices. Asian J. Biol. Sci 2023, 16, 600-613. https://doi.org/10.3923/ajbs.2023.600.613
AMA Style
Akpaetok
DE, Ahaotu
I, Maduka
N. Effect of Pasteurization and Storage Temperatures on the Quality of Carrot Juices. Asian Journal of Biological Sciences. 2023; 16(4): 600-613. https://doi.org/10.3923/ajbs.2023.600.613
Chicago/Turabian Style
Akpaetok, Daniel, Enefiok, Ihuoma Ahaotu, and Ndukwe Maduka.
2023. "Effect of Pasteurization and Storage Temperatures on the Quality of Carrot Juices" Asian Journal of Biological Sciences 16, no. 4: 600-613. https://doi.org/10.3923/ajbs.2023.600.613

This work is licensed under a Creative Commons Attribution 4.0 International License.


