Molecular and Epigenetics Mechanisms for the Immune Control of Plasmodium Parasites Infection: A Comprehensive Review
| Received 16 Oct, 2023 |
Accepted 30 Nov, 2023 |
Published 31 Dec, 2023 |
Malaria is a vector-borne infection common in tropical and subtropical countries. The immunological reactions warranted by the malaria Plasmodium falciparum parasite occur with multiple features, as seen in the human host and in the Anopheles mosquito. Numerous methods using phytomedicines and molecular strategies exist for disruption of Plasmodium transmission. Herein, the molecular and cellular basics occurring in the vector and host immune response are explicitly discussed with a view towards effective drug targeting. A key area of interest to target for vaccine development is the CD4+ T helper cells production of proinflammatory cytokines that activate macrophages, thus promoting the activation of specific B cells as seen in the erythrocytic stage where the action of CD8+ T cells is thought to be insignificant. Similarly, the γδT and NK cells with IFN-γ, perforin and granzyme produced being implicated in destroying RBCs infected with P. falciparum. Moreover, some genetic markers such as the Dantu red blood cell variant, ABO blood group system, hemoglobinopathy, glucose 6 phosphate dehydrogenase deficiency, etc., associated with natural resistance to malaria parasites are also espoused. Through the course of human development, immune response to various toxicants, shown some elasticity towards microbial exposures, driving epigenetic modifications allowing innate immune cell programming. Malaria co-infection remains an issue, evidence suggests malaria and HIV infections, for instance, undergo bidirectional and synergistic interaction. Moving forward, some important biological receptors such as ferriprotoporphyrin, the involvement of anti Plasmodium IgG, SMI peptide and cyclotide antimicrobial peptide, regulate Plasmodium parasite immune response in the mosquito and possibly human host and the interaction of complement factors may bring about the immunological reaction to infection with Plasmodium. The incorporation of different clinical, genetic markers and epigenetic factors may help establish utility that may bring about a novel control mechanism involving molecular and epigenetic properties for malaria disease.
| Copyright © 2023 Adejoh et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Malaria is a widespread infection in subtropical and tropical areas and an important infectious diseases the world over having an immense rate of mortality and morbidity among children and adults1. Recently, there have being an upward surge in the epidemiological burden of such severe life-threatening diseases like, malaria, cancer, typhoid, diabetes, hepatitis, Human Immunodeficiency Virus (HIV), etc., on humans all over the world. This has mandated clinicians and researchers to develop meaningful therapeutic strategies for such diseases2,3.
The convoluted biological life cycle of Plasmodium species and the emergence of the parasite variants gave the inkling for the improvement of various measures t that can disrupt completion of plasmodium life cycle in both the vector and human host4. Numerous methods are presently undergoing experimentation in the area of disruption of Plasmodium transmission, using phytomedicines and molecular strategies3,5. Genetics and molecular control tools/strategies including; interruption of the Plasmodium cell surface receptor protein, Feline Leukemia virus subgroup C receptor (FLVCR) thought to be involved in transporting heme out of the cell are needed, to prevent the interaction between the Plasmodium thrombospondin-related adhesive protein (TRAP) with the Anopheles Saglin protein, aiding the malaria parasite for the invasion of the mosquito salivary gland. Further, gene silencing-techniques are also required to target the the mosquito gut leading to reduction levels of FLVCR. This is with a view to possibly promote the prevention of the interaction of surface enolase and plasminogen of mammalian blood, enabling the disruption of an important role in ookinete invasion of the mosquito midgut. Thus, the use of plants with cysteine base protease inhibitors and antimicrobial peptides (cyclotides), with characteristics structural similarities to SM1 peptide, an inhibitor of Plasmodium TRAP-saglin binding; including the use of phyto-active compounds to block Plasmodium transmission have all been suggested as some possible ways and options for the elimination of the dreadful disease4,5.
Undisputedly, in comparison with other Plasmodium species, Plasmodium falciparum is culpable for most of the deaths (99%) due to malaria6,7. The virulence and the skills of the parasite’s to evade the vector and human immune system through different mechanisms have been implicated as responsible for its immune feedback6. This review focuses on the molecular and cellular immune processes involved in Plasmodium control, externalized by; intonation of anti-Plasmodium immune responses by host genetic factors, regulation of anti-Plasmodium immune feedback by environmental factors, synergy between Plasmodium infection and other extraneous pathogens during co-infections, the fallout of Plasmodium infection on the host global immunity and on the attendant diseases and its implication for the development of novel treatment strategy.
In this review, the molecular and epigenetic factors underlying plasmodium activities and the required immune response were provided.
Literature review
Molecular and cellular immune processes employed in the control of Plasmodium: Immune response against the P. falciparum malaria parasite occurs with multiple features with stage specificity, seen in the human host and the Anopheles mosquito vector7. Moreover, immunological responses have been recognized as a source contributing to the pathophysiology of the disease in humans7. Herein, the molecular and cellular mechanisms involved in the host and vector immune feedback were explicitly discussed.
Anopheles mosquito’s immune response: Numerous factors including: The immunological, microbiological and physical defenses of the vector immune system are known to affect the Plasmodium falciparum malaria parasite before constituting infection in its Anopheles mosquito vector8. Immunological defences are thought to play some key roles in the Plasmodium biological cycle, especially, during the transmission of ookinete to the mosquito midgut and the sporozoite’s migration to the salivary glands4,9.
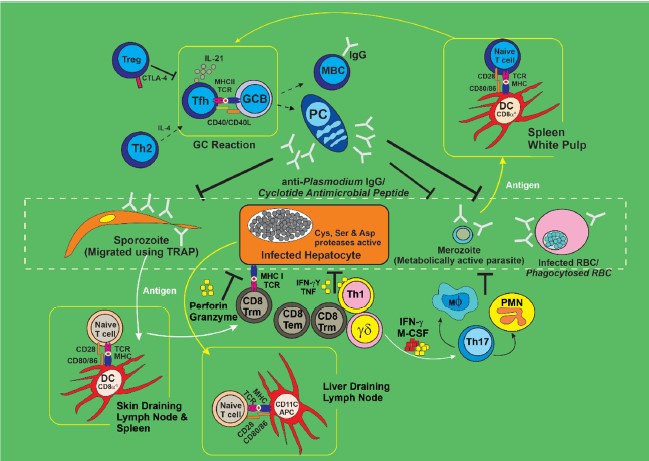
|
Using the TRAP, the sporozoites traverse into the mosquito salivary glands with an adhesive domain (‘A-domain’), a protein recognized as Anopheles saglin protein, whose structure has been studied and found to possess some special motif for the binding of cyclic cysteine peptides such as SM1 peptide and cyclotide antimicrobial peptide (Fig. 1)10. This offers great potential for the development of anti-plasmodial therapy10. The SM1 peptide and cyclotide antimicrobial peptide are thought to possess some cysteine disulfide bridge(s) referred to as cysteine knots, recognized by Anopheles saglin protein motif, enhancing their covalent bond binding potential to these proteins4, with concomitant binding of these peptides to saglin protein preventing sporozoite-TRAP binding, thus abrogating the transmission of sporozoites to the mosquito salivary glands. This interaction was suggested to enhance immune control of the Plasmodium parasite in the vector7 bearing the mosquito immune system reaction is paramount in the control of its vectorial capacity which is implicated in several malarial disease cases11.
Physical barriers: In the propagation of malaria, the Anopheles mosquito’s first line of defense against the parasites P. falciparum is its physical barrier. A major physical barrier to P. falciparum, offering some protection to mosquitoes against ookinete infection is the peritrophic membrane (PM) of the mosquito midgut; so are the cuticle of the exoskeleton (lining with membrane vesicles), as well as the epithelial cell lining of the tracheal respiratory system7. Also, the formation of the capsule by mosquito melanin around the parasite has been found to provide some protective role against the parasite infectivity11.
Midgut microbiota: The Anopheles mosquito’s microbiota such as Asaia, Enterobacter, Pseudomonas and Pantoea12, found in it’s midgut is critical for the immune feedback against Plasmodium (ookinete) because they are connected with the potential of inducing adenosine monophosphates (AMPs), which spur the basal inherent immune action against P. falciparum infection13. Moreso, earlier reports were ‘an increased susceptibility to Plasmodium malaria infection in microbe-free mosquitoes’14, further indicating their importance and protecting the vector immune systemic response.
Humoral immune response: The Anopheles mosquito humoral immune feedback against Plasmodium parasites is characterized by the mosquito hemolymph which contains proteins that include, complements such as or Thioester-Containing Protein (TEP1)13. These proteins form leucine-rich repeat protein 1 (LRIM1) producing complexes with anopheles Plasmodium-responsive leucine rich repeat protein (APL) 1/TEP1, which accumulate on the ookinete surface for phagocytosis13. Important players are apolipoprotein D precursors, apolipophorin and fibrinogen-related proteins with potential antiplasmodial defense within the vector midgut13. In concordance, is an existing report that hemozoin activates the transcription of several key immune genes such as REL2-F (transcription factor)15, regulating other important proteinse e.g., FBN9, LRRD7, APL1 and TEP1 as anti-Plasmodium immune factors16.
Cellular immune response: The hemocyte cells contained in hemolymph are the key immune cells involved in pathogen recognition, binding fat body for the management of immune peptides and coordination of the mosquito intrinsic immune feedback17,18. These cells include the prohemocyte subtypes, oenocytoids and granulocytes, which partake in the process of phagocytosis, melanization and hematopoietic cells precusor, respectively13,19. Further, LPS-induced TNFα Transcription Factor (LITAF)-like 3(LL3) influences oocyst survival and hemocyte differentiation18; some other immunological effectors released by hemocytes and fat body into hemolymph are believed to engaged in phagocytosis, encapsulation, secretion of antimicrobial peptides, agglutination, nodule formation and melanization7. All of this, coupled with the reactive oxygen species (ROS) also manufactured by hemocytes play some important roles in mosquito immunity against P. falciparum7,20,21.
Vector anti-plasmodial immunity signaling pathways: Antiplasmodial immunity signaling molecules in the mosquito, such as Janus Kinase (JNK), immune deficiency (Imd), the toll and signal transducers and activators of transcription (STAT), are thought to add to anti-Plasmodium defense and also to be involved in theantiplasmodial signaling pathways. In the Plasmodium parasite biological cycle, the Imd and the Toll pathways target the ookinete stage of the parasite stimulating the action of the mosquito TEP1 complement-like system, which stimulates the antigen secretion in the midgut11,22. Recognition of the Pathogen-Associated Molecular Patterns (PAMPs), activates these pathways and NF-κB, leading to the activation of Rel1 and Rel2 in Toll and Imd pathways, respectively22. These activations are thought to be paramount for the entry of adenosine monophosphates (AMPs) into the nuclei as with defensins, cecropins, attacin and gambicin, possessing antiplasmodial activity13. The Rel1 and Rel2 are controlled (negatively) by regulators, Cactus and Caspar, respectively in Anopheles species and also the Imd pathway control anti-Plasmodium effectors e.g., LRRD7, APL1, FBN9 and TEP1, thought to be potent players20. Moreover, the immune-enhanced Anopheles stephensi mosquitoes using Rel2 in the midgut exibited better resistance to Plasmodium infection, giving a clearer direction that may lead to designing appropriate control strategies13,14. More studies in this line have thrown up a putative biomarker such as the activation of the Imd and Toll pathways that induce the expression of AgDscam (Anopheles gambiae Dscam receptors) isoforms that have species-specific antiplasmodial responses23.
Another important signaling pathway, the JNK-STAT pathway, has been linked with anti-Plasmodium defense, even though; the details of the activation have not been fully understood13. However, the expression of HPX2, NOX5 and TEP1 in hemocytes, promoting TEP1-intercession lysis is regulated by the JNK pathway23.
Molecular signal transducers such as the STAT1/AgSTAT-B and STAT2/AgSTAT-A that are also activators of transcription genes are shown to mediate immunity against the P. falciparum malaria parasite. Specifically, the transcription gene AgSTAT-A is required in the transcriptional activation of nitric oxide (NO) synthase that increases reactive NO leading to the transcription of suppressors of cytokine signaling (SOCS), with a negative effect on parasitic development7.
Immune response targeted at the Plasmodium falciparum in human: The immune response to P. falciparum in human is complicated and it is targeted at different phases of the biological cycle of Plasmodium parasites. The involvement of the immune response is high in the erythrocytic phase in contrast to the pre-erythrocytic stage and this is because of the several metabolic activities occurring during the formation of merozoites. The major immune players at the stages of the pre-erythrocytic and erythrocytic include the CD8+ T cells and antibodies24.
Skin as physical barrier of the human host: The skin serve as the first important physical barrier in humans to the invasion of Plasmodium parasite, they remain the first line of defense for many pathogens including P. falciparum malaria parasites. Following inoculation, sporozoites are in the skin for several hours before they are primed or tiggered into a state of readiness for the hepatic phases. Some critcal antibodies found in human skin deter sporozoite movement in the dermis25. Therefore, about 50% of the sporozoites do not leave the primary injection site26, consequently, monitoring the early stage of this inoculation could be a key target in vaccine development15.
To penetrate through the skin barrier, including cell traversal, with subsequent migration to the liver, two important sporozoite proteins (SPECT1 and SPECT2), are found necessary27, bearing they prime sporozoites to evade destruction by phagocytes and their arrest in growth in nonphagocytic cells in the human dermis7,28.
Immune response targeted at the Plasmodium parasites pre-erythrocytic-phase: For immune response, the pre-erythrocytic phase is one of the targets of sporozoites and infected hepatocytes. Thus, to block the invasion of hepatocytes, antibodies against free sporozoites and circumsporozoite protein (CSP) are necessary for neutralizing those proteins that are needed for cell traversal and occupation. These antibodies activate immune response via the complement fixation, phagocytosis and lysis by cytotoxic NK and NKT cells. Also are some neoantigens present at the surface of infected hepatocytes, which pass through an antibody-dependent cell-mediated mechanism by Kupffer cells and NK cells7. These neoantigens are mainly involved in the phagocytosis of intrahepatic parasites via the CD8+ T cells lymphocytes producing gamma interferon (interferon-γ). Other cells (aside CD8+ T cells) like NK, NKT and γδT cells which destroy intrahepatic parasites through secretion of type I interferons and IFN-γ24,29-31, are also involved in this process.
Malaria parasites exemplified by P. falciparum, unlike bacteria and viruses, trigger type I IFNs in the absence of Toll-like receptors (TLR3 and TLR4), through their signaling proteins (MyD88 and TRIF). They can use a melanoma differentiation-associated gene 5 protein (MDA5) and communicate through some activators of the transcription factors IRF3 and IRF7, which are known mitochondrial antiviral signaling protein (MAVS)30. An exoerythrocytic-form (EEF) RNA is recognized by MDA5 in hepatocytes and such reaction is thought to trigger a type I IFN response in the innate immune cells28,32. These processes (disruption of infected hepatocytes and prevention of invasion by CD8+ T cells and antibodies) are important targets for vaccine development33.
Immune response targeted at erythrocytic phase of Plasmodium infection: The immune response targeted at the erythrocytic stage of P. falciparum is more complicated than other phases of immune responses34. This immune response against Plasmodium infection is initiated by the release of merozoites from hepatocytes leading to RBC invasions and this interaction is mediated by proteases rich in conserved cysteine, serine and aspartate residues5,7,35. In controlling both the merozoites and intra-erythrocytic parasites, the humoral or antibody and T cell responses are relevant. This is a good target point for drugs/vaccines. As a fact, antibodies have the ability to convert merozoites to foreign cells for phagocytosis or inhibition of invasion of RBCs. Specific monoclonal antibody is known to mediate cell death, prevent the union of infected RBCs to the endothelium and neutralizes parasite toxins, thereby, blocking excessive inflammation induction7, marking merozoites for lysis via the complement system36, through the proinflammatory cytokine response that activates macrophages during the immune response to viral infections30.
The part played by CD8+ T cells in the erythrocytic phase is believed to be minute34, while that of the CD4+ T helper cells is to produce proinflammatory cytokines that activate macrophages37, promoting the activation of specific B cells, a target point for vaccine development38,39. In addition, NK and γδT cells27 with IFN-γ, perforin and granzyme produced by NK cells are responsible for destroying RBCs infected with P. falciparum7.
Gametocyte targeted immune response: Gametocytes are eliminated by antibodies through complement-mediated lysis. The antibodies also prevent the maturation and sequestration of gametocytes in the host. The complement-mediated death of gametocytes is promoted by antibodies gotten during a blood meal from the host. It also prevents mosquito gamete fusion. It should also be noted that nitric oxide produced by macrophages partakes in the killing of gametocytes27.
T cell subgroups control of resistance to Plasmodium infection in the host: The presence and function of γδT cells, cytotoxic CD8+ T cells and helper CD4+ T cells are fingered for the response to both the symptomatic blood-phase and the asymptomatic liver-phase for Plasmodium parasites infection in malarial disease immune response24. Regulatory T cells are believed to mediate these T cell responses but the exact mechanisms and process in the development and function of Plasmodium-specific T cells and the process to form tissue-resident and long-lived memory populations are not clear. Figure 1 presented an overview of tissue-specific, T cell-mediated immune resistance networks during Plasmodium infection.
The parasite (in form of sporozoite) migrates into mosquito salivary glands using sporozoite-TRAP. This protein is recognized by Anopheles saglin, allowing binding to enhance transmission and subsequent infection, upon mosquito bite on the host. The parasite traverses through the host blood into the liver subsequently infects the hepatocyte. Some important Plasmodium proteases like cysteine, serine and aspartate protease were identified to be actively involved in hepatocyte infection. The metabolically active merozoites break out of the liver, thereby infecting/phagocytosing the RBC. In order to control the parasite transmission, we have identified cyclotide antimicrobial peptide (similar to anti-Plasmodium IgG) with the potential of preventing sporozoite-TRAP and saglin binding. In addition, cysteine and serine-based peptides found in some plants (e.g., Calotropis procera) and bacteria (e.g., marine actinobacteria) have been identified as potent inhibitors of Plasmodium proteases involved in hepatocyte.
Anti-Plasmodium immune response modulation by host genetic factors: Several factors including pregnancy, mixed-infections with other pathogens, nutritional status, age and host genetics may influence the evolution of clinical immunity to malaria infection40-42. In light of the prevalence of some genetic disorders in malaria-endemic areas, conferring protection against the dreadful disease, the importance of some human genetic biomarkers responsible for the immune response to malaria is being suggested (despite its general disadvantages).
A human genetic factor seen with natural resistance to malaria parasites, the Dantu polymorphism, is a powerful protective alternative of red blood cell membrane proteins like glycophorins, are found most commonly inhabitants of the Kenyan Coast43. Concerning the regulation of anti-plasmodium immune responses, others that have gained attention include glucose-6-phosphate dehydrogenase mutated genes, blood group O+, IgG immunoglobulin, alpha thalassemia, FcγR gene polymorphisms, Human Leukocyte Antigens (HLA), hemoglobinopathies, ethnicity and mutation at glycophorin gene cluster resulting in the presence of two GYPB-A hybrid genes, encoding the Dantu blood group variant43,44.
The Dantu is believed to inhibit malaria infection by changing the biophysical structure of red blood cells (hosts to the parasites), thereby conferring protection against the disease and it is further thought that it may provide a wider health implication in the quest for control of malaria43.
Red Blood Cell (RBC) variants: The human Red Blood Cells (RBCs) are very important in the life cycle of the malaria parasite. The parasite utilizes the RBC in different forms, including as shelter and as food sources. However, the pathogenesis of the disease is an offshoot emanating from the interaction between the infected RBCs and uninfected RBCs and other tissues. Consequently, molecular alterations in the RBC structure or function will interfere with RBC invasion by the parasite and/or parasitic growth and multiplication inside the RBCs and this consequently affects the disease phenotype. Below are discussed some RBC variants found to be important in malaria immune process.
Dantu red blood cell variant: Malaria is amongst the most dreaded diseases in the world with a major effect on the human genome, with some protective polymorphisms such as the sickle-cell trait-exibiting high frequencies in malaria-endemic regions45,46. The Dantu variant gives about 74% protection against severe malaria in homozygous individuals47-49, which in relative terms, this blood group confers a similar degree of protection as the sickle-cell trait and is believed to be greater than that conferred by the best malaria vaccine. The Dantu effect is seen with wide changes to the full range of proteins, such as anion transport protein (band 3) and glycophorin A, B, C and D found on the RBC surface; this mechanism was only recently demonstrated as the protective effects of Dantu on the ability of the merozoite form of the P. falciparum on RBCs43. Surprisingly, the inhibition of invasion was found not to correlate with specific RBC–parasite receptor-ligand interactions43. Video microscopic examination of Plasmodium parasite invasion indicates a strong link between RBC tension and merozoite invasion, with a tension threshold above which invasion rarely occurs, even in non-Dantu RBCs43. Dantu RBCs were noticed with higher tension than non-Dantu RBCs, indicative of why this blood group possesess a greater proportion of resistance to invasion. This knowledge gives insights into why the efficiency of P. falciparum invasion might vary across the heterogenous populations of RBCs found within and between individuals and the protective effect of Dantu43. It suffices to mention RBC surface glycophorins carry the antigens of the Rh blood group system, blood group ‘N’ antigen and the Ss antigens, as well as the Gerbich antigens50.
ABO blood group system: The ABO system, the most medically important RBC polymorphism has on the surface of erythrocytes structural polymorphism in the carbohydrate moiety as the basis of their classification51. There has been a corrolary between the ABO system and the severity of malaria and correlations between the disease and the ABO blood groups52. The blood groups A, B and AB form rosettes (binding of Infected RBCs (iRBCs) to uninfected ones and endothelial cells specifically the endothelial protein C Receptor), linked to severe disease occasioned by P. falciparum. These may have been caused by some promiscuous lectin-like interactions, happening more readily in these blood group subtypes than with blood group O41. Following, persons with blood groups A, B and AB are more likely to have cerebral malaria compared to those with blood group O41.The ratio of blood group O to non-O is greater than 1 in malaria-endemic areas, whereas this ratio is smaller than 1 in other areas51,53. There is yet to be a linkage of the ABO system to the frequency, prevalence and or anti-malaria antibody levels52.
Haemoglobinopathies: Hemoglobin (Hb) an iron-containing protein complex found in RBCs helps in the transportation of oxygen from the lungs to other parts of the body54. The most common human genetic disorders of Hemoglobin (Hb) structure and production are called hemoglobinopathy (associated with sickle-cell disease and thalassemia). The Hb molecules consist of four polypeptide chains called “globins”, having 2 alpha-chains (containing 141 amino acid residues) and 2 beta chains (containing 146 amino acid residues) and an iron-containing molecule, called haem, attached to each chain55.

|
Two categories of hemoglobinopathies exist, qualitative and quantitative hemoglobinopathies. Qualitative hemoglobinopathy is associated with the quality of hemoglobin in terms of variation in Hb structure, e.g., a sickle-cell disorder such as HbS, C and E. But quantitative hemoglobinopathy, concerns the amount of hemoglobin produced being affected and leading to formation of normal α or β-globin, causing α and β-thalassemia, respectively56. Knowledge of these hematological disorders especially during the invasion of the human RBC by the merozoites gives some useful options in the management of malaria episodes55. It is obvious that merozoite invasion of the RBCs and subsequent degradation of the hemoglobin are impaired by interactions involving the formation of sickle-cell and thalassemia, a reaction which is thought to prevent merozoite multiplication and further invasion.
Sickle cell disease (SCD): The SCD is a genetic disorder occurring from the point mutation in the β-globin subunit of hemoglobin57. Figure 2. Presents cases of haemoglobinopathies including SCD. Hemoglobin S is formed when two wild-type α-globin subunits located on chromosome 16 are associated with two mutant β-globins on chromosome 1141,57. Normal RBCs contain HbA and are elastic, enabling the cells to buckle in order to pass through capillaries but HbS-carrying RBCs are distorted and tend to lose their elasticity, also they have shorter lifetimes as compared to the normal ones58. The homozygous form of HbS (HbSS) causes early childhood lethal severe disease, in the absence of adequate treatment. The heterozygous form (HbAS), referred to as the sickle-cell trait, is connected to the protection from severe malaria59 in them.
A number of processes by which SCD mediates resistance to malaria abound60,61. Among these are enhanced phagocytosis of ring-phase parasitized HbAS erythrocytes, impaired intra-erythrocytic parasite growth in AS-RBCs and reduced erythrocyte invasion, as compared to infected normal erythrocytes62. The HbAS-parasitized erythrocytes also exhibit sickling, which may promote the removal of premature infected erythrocytes in the spleen61, another finding is enhanced immune responses to parasitized HbAS erythrocytes41. There is a reduction in the binding of P. falciparum-infected AS erythrocytes to endothelial cells of the microvasculature and blood monocytes as compared to infected normal RBCs63 which may serve as a useful candidate in protecting against severe malaria conferred by the sickle cell trait. Therefore, the exact process that SCD protects against malaria is needed to understand the various pathways in order to have a concerted view of the management of malaria infection. Parasite’s ability to stick to both CD36 and EPCR in vitro is reduced by HbAS, attenuating the expression of parasite proteins (PfEMP1) on the erythrocyte surface61. Sickle cell trait reduces the surface expression and function of PfEMP1 and this provides a direct process for protection against severe malaria61.
Alpha-thalassemia: Located on chromosome 16, the human RBC haemoglobin-alpha-chain gene (usually 2 on each chromosome) has 4 α-chains in total58. The production of alpha-chain protein is squarely shared by the four genes58. The genetic and hematological disorder involving HbA1 and HbA2 genes (alpha-thalassemia) occursvia impaired interaction on chromosome 16 leading to the deletion of a gene(one or more genes mutate) or failure to performcausing underproduction of hemoglobin64,65. In Alpha-thalassemia there also may be unstable beta-globin molecules, leading to an increased RBC attenuation65. The type and severity of the disease is controled by the number of genes deleted (ranging from four to one deleted gene) either on the same chromosome (cis deletion) or a different chromosome (trans deletion)64.
The α-thalassemia provides consequential protection against severe and mild malaria55,66. Also, α-thalassemia disturbs the cytoadherence of parasitized RBCs to endothelial microvascular cells and monocytes, these interference are pivotal in the pathogenesis of severe forms of malaria67.
Further, α-thalassemia causes diminished expression of the red-cell complement receptor 1 (CR1), a crucial receptor for rosetting67, implying that impaired CR1-moderated rosetting may provide resistance to severe malaria occurring in α-thalassemic patients.
Glucose-6-phosphate dehydrogenase deficiency: The X-linked recessive disorder, Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency, is caused by a deficiency of the ‘housekeeping’ enzyme (with antioxidant capacity) protecting the cell from oxidative damage. Several variants (up to 442) of the gene (G6PD) located on the long arm of the X chromosome exist. As 130 of the 442 variants have been localized at the DNA and found to impair enzyme activity68,69. The down regulation of this enzyme in the RBCs is fatal68. Mutation in this enzyme (usually resulting from exposure to infections, reaction to certain foods or medication) can lead to anemia70.
Malaria parasites break down hemoglobin inside the RBC, with the resultant production of oxidized iron, which harms the parasite. Any of the defects in G6PD function, which is paramount for cells to overcome oxidative stress, are toxic for the parasite and can therefore confer resistance to malaria infections70,71. The mutant G6PD-A variant is called the Mahidol gene and have an approximate function of 10-50% as compared to the wild enzyme activity, this variant is shown to be the most common G6PD deficiency allele in Sub-Saharan Africa41,71.
The effect of the common G6PD-Mahidol (487A) variant in Southeast Asia on human survival in relation to P. vivax and P. falciparum malaria71 indicates that strong and positive selection affected the Mahidol variant over the past 1500 years. The same variant reduces P. vivax parasite density in humans but had no such effect on P. falciparum’s (parasite density), implying P. vivax is the force behind the strong selective advantage proffered by this mutation. Though the exact mechanism by which G6PD conferred protection against P. vivax-induced infection is not known, the reduction in parasite density by G6DP deficiency is thought to be the increased responsiveness of P. vivax to oxidative stress, knowing that G6PD deficiency leads to increased oxidative stress in RBCs, which have a negative impact on the parasite, conferring some protection against malaria on individuals with this mutated gene70,71.
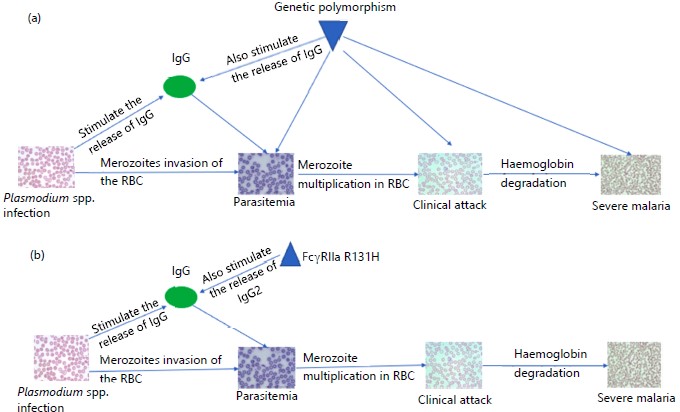
|
Other host genetic factors that affect anti-Plasmodium immune response: Molecular pathways regulating genotypes with phenotypic outcomes represent the effectiveness of host immunity, notable among these are parasitemia and IgG levels, which are paramount, owing to that effective vaccines and new treatments are presently sorted. The genetic basis of parasitemia levels and IgG levels shows that the protective effect of malaria clinical phenotypes may partially be moderated via parasitemia and cytophilic IgG levels72. Figure 3 shows a simplified diagram of Plasmodium spp., infection to development of severe malaria. The IgG receptor FcγRIIa, encoded by FCGR2A is a typical example bearing, H131 homozygotes displayed higher IgG2 levels and are protective against high parasitemia and onset of malaria symptoms72. Other genetic disorders such as Duffy and Gerbich blood-group antigen deficiencies, CR1 polymorphism and Southeast Asian Ovalocytosis (SAO) are some other examples of RBC variants, involved in malaria resistance41.
Diagram A and B modified from Louicharoen et al.72 and adopted for this review illustrate the role of IgG and IgG2-mediated acquired immunity in Plasmodium infection. In both diagrams, parasite invasion of the RBC results in parasitemia, where the RBC is exposed to attack by merozoites. This is enhanced by the utilization of Plasmodium proteases such as cysteine, serine and aspartate proteases. The clinical attack is marked by merozoite multiplication in the RBC, leading to severe malaria when the parasite infestation of the RBC and subsequently degrade the hemoglobin. The degradation of hemoglobin brings about oxidative stress seen in most cases of severe malaria.
Human Leukocyte Antigen (HLA): The Human Leukocyte Antigen (HLA) is a complex of genes on chromosome 6 in humans. This system or complex in humans encodes cell-surface proteins, responsible for the regulation of the immune system and demonstrates a potential immune response to malaria infection73. The HLA is of the Major Histocompatibility Complex (MHC) gene loci in humans and they are highly polymorphic. Several pieces of evidence show that specific HLA alleles might cause susceptibility/resistance to malaria disease74. Some studies have shown HLA class I antigen, HLA-B*5301 and HLA class II haplotype, HLADRB1*1302-DQB1*0501, to possess potential resistance to severe malaria, as evident in Gambian malaria75. The HLA subclass, “HLA-DQB1*0501” is linked to a drop in risk of re-infection and anemia in Gabonese children75, while DRB1*04 and DPB1*1701 were implicated for severe disease74. An association between HLA-DRB1*04 and severe malaria was clearified by the case-control study in Ghana76.
Ethnicity: A study was carried out on malaria in Kenya showed that 25% of malaria variants are due to host genetic factors; implying higher ratio far beyond the effects expected from the hemoglobinopathies. Also, the same study, showed that the HbS gene, which is the strongest known genetic resistance factor to malaria Plasmodium, amount to only 2% of the total variants. This findings and others supports other suggestions that there may be a large number of protective genes present in the population, conferring some degree of protection41,77,78.
Differences in malaria susceptability due to ethnic factors offer a useful approach to identifying immune genes implicated for the resistance or susceptibility to malaria in Africa. Such reasoning is supported by a study in Mali, which revealed some significant inter-ethnic variation in susceptibility level79. Furtherance, the Fulani ethnic group in Sub-Saharan Africa (SSA) are not prone to develop clinical malaria infection compared to other ethnic groups, such as the Dogon in Mali, the Mossi, Rimaibe in Burkina Faso and the Masaleit in Sudan, despite seeming exposure to the same parasite and, same transmission intensity. Such findings is suggestive, the Fulanis, have higher anti-malarial-immune reaction and lower asymptomatic infection prevalence than other Africans80. Evidently, this relative resistance to malaria in this ethnic group seems not to be correlated to classical malaria-resistance genes, bearing higher prevalent of α-thalassemia, G6PD, HbC and HLA-B*5301 were found in the Mossi and Rimaibe, compared to the Fulani. Similarly, in Mali, HbC was more frequent in the Dogon group than with the Fulanis, with HbS showing low similar occurance in both ethnic groups79. The study comparing Fulani and other Africans also reported a significantly higher frequency of HbAS in non-Fulani (Masaleit) compared to Fulani80.
In addition, a study in Burkina Faso showed an increased in Peripheral Blood Mononuclear Cells (PBMC) from the Fulani compared to the Mossi in both Th1 (IL-18 and IFN-γ) and Th2 (IL-4 and GATA 3) related genes. However, the Fulani had a reduced expression of Foxp3 and CTLA4. Which are Treg determinant genes. These results suggests, that the defect in functional genes encoding for Treg in the Fulani ethnic groups, may have been responsible for their higher resistance to malaria81. These results are supportive of reports from Mali, showing a more efficient Th1 and Th2 response towards P. falciparum antigens in the Fulani ethnic group82. However, in the protection against malaria, different factors including epigenetic makeup might be a major contributing factor.
The human genetic variations and sympatric ethnicity differences in the immune response to malaria disease in Africa wereidentified among the Fulani group and the Dogon in Mali. Figure 4 simplified the activation of DC leading to IFN-γ and IL-18 (Th1) normal TLR responses. Potent T cell activation enhances humoral immunity/ defense against the Plasmodium parasite. This was due to the release and activation of CD4+ and CD8+ T cells which offer protection to the Fulani group against malaria disease. On the other instance, among the Dogon, DC activation is impaired leading to impaired TLR responses. This, however, bring about poor T cell activation, unlike the Fulani group where a potent T cell is activated. Poor T cell activation impaired humoral immunity and as a result, Plasmodium infection enhanced.
FcγR gene polymorphisms: The human Fc-Gamma Receptors (FcγRs) polymorphism influences malaria susceptibility and pathogenesis78. In the context of the many candidate genes (complex genes clusters), the Fc Gamma Receptors (FcγRs) for IgG are the most extensively studied out. The FcγRs for IgG is possesed by a variety of immune cells such as leucocytes and monocytes. Typically the FcγRs receptors bind to opsonized pathogens towards activating a variety of cellular immune responses that may climax in the control of infection82. Thus, these complex genes clusters are thought to be an important connection between humoral and cellular immune response78. A study reported that three families of human FcγRs with Single Nucleotide Polymorphisms (SNPs) (FcγRIIB-rs1050519, FcγRIIC-rs3933769 and FcγRIIIA-rs396991) have been found amongst sympatric Fulani and Dogon children with resultant uncomplicated malaria78. Each FcγR family constitutes a different type of isoform. Thus, such isoforms as theA-, B- and C are referred as FcγR-I and II, whilst FcγRIII includes two isoforms, A and B. These isoforms manifests on a variety of cell types and vary in relation to their binding affinity to different immunoglobulin G (IgG) subclasses78.
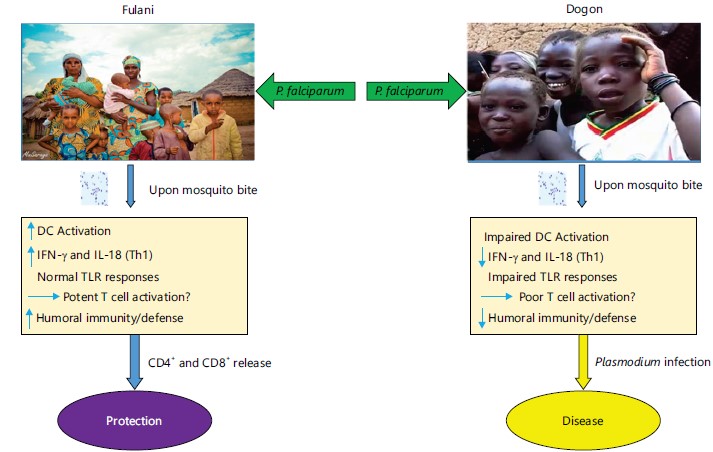
|
Many studies have shown FcγRIIA-G494A83,84, FcγRIIB-T695C85 and FcγRIIIB-NA1/NA286), which are FcγRs gene polymorphisms to enhance susceptibility to and/or severity of malaria. A point mutation such as the G494A in the FcγR IIA gene leads to an amino acid exchange, arginine (R)/histidine (H) at position 13187. This FcγR IIA-131H mutation, is the only human FcγR that coherently binds the IgG2 subclass, controling the immune response to the disease in a synchronized manner87. Different studies on the H allele suggest it offers both protection and susceptibility to malaria78. For instance, studies in the Sudan and Mali reported significantly higher frequencies of the H allele and the H/H genotype in the Fulani as compared to the non-Fulani84. However, there is still no adequate evidence to support the proposition that the FcγR IIA131H allele plays a key role in reducing the susceptibility to malaria disease in the Fulani. Thus, this will offer a useful opportunity to research on malaria control by regulating to our advantage some of these human genetic factors.
Cytokine gene polymorphisms: The immune response against Plasmodium parasitic variants such as Plasmodium vivax to elicit some epitopes are balanced by pro- and anti-inflammatory cytokines which regulate antibody levels and class switching in malaria treatment88. Individual differences in the production of cytokines involved in malaria immunity may influence the course of infection and the disease outcome therefore, the humoral immune response may be partly regulated via cytokine gene polymorphisms88. Genetically, some the cytokines can regulated through some processes involving the introduction of polymorphisms in cytokine-related genes to enhance their capacity to produce variable amounts of cytokines that can modify or downregulate cytokine expression and/or function88. For example, the substitution of a nucleotide C by a T at -590 upstream position in the IL-4 gene promoter has been described to upshot in enhanced IL-4 production89. Similarly, a replacement of nucleotide G to A substitution in the IL-10 gene upstream promoter, position -1087, has been identified to elevate the quantity of IL-10 assembly88.
Some studies have established the occurrence of polymorphisms in cytokine-related coding genes to various diseases, including malaria. Thus, an increased levels of TNF-α for instance, in cases as cerebral malaria patients are a consequent effects emmanating from variants in the TNF-α promoter of the individuals90. For patients with malaria of Thai origin, the level of IL-13-1055 T allele has been shown to protect against acute malaria88, whilst the IL-4-590 T allele, elevate IgE levels in asthmatic children90. In Burkina Faso, same allele was shown to be associated with increased malaria-specific IgG levels in persons of Fulani ethnic stock78. Even though, there seems to be no reported significance differences in the frequency of T cell allele occurance in severe and mild malaria cases89. Polymorphisms in the Interferon-Regulatory Factor (IRF-1) have recently been linked to the control of P. falciparum infection88, although none of the studied polymorphisms detailed in most reviews has yet been suggested as a determinant of susceptibility/resistance to malaria90,91.
Environmental factors in the regulation of anti-Plasmodium immune response: There tend to be acquired immunity to Plasmodium infection by humans following repeated exposure to malaria parasites, this leads to clinical protection with a reduction in erythrocytic stage infection with life-threatening parasitemia and in addition, there is a high level of immunoglobulin G (IgG) involved in adult malaria immune response in malaria-endemic countries80. Thus, the increased antibody levels against blood stage merozoites antigens is of importance in clinical protection against malaria, hence, it should be an area to harness for potential anti-malaria vaccine91-94. Additionally, protective immunity has been attributed to the high concentration of IgG95. Furthermore, anti-Plasmodium falciparum antibody increases and peaks 1-2 weeks following malaria infection with a rapid decline thereafter, indicating a short-lived duration of circulating IgG to the merozoites antigens96. Antibody response may last for many years as a consequence of age concerning Plasmodium exposure and concomitant environmental factors that potentiate antigen-specific antibody response to Plasmodium infection; these factors may be paramount in choosing antigens for the development of anti-malaria vaccine91.
Previous exposure to the Plasmodium pathogen, parasite density, genetic factors, co infection and nutritional status coupled with environmental factorsmay influence cytokine production97. In malaria-endemic countries, the human population is frequently exposed to the salivary component of blood-feeding mosquitoes which possess a variety of pharmacological active biomolecules with anti-hemostatic, anti-inflammatory and immunomodulatory properties97. Mosquito immunomodulatory salivary proteins (Anopheles saglin protein) act both on innate and adaptive immunity by stimulating the T and B cell proliferation which are found to be susceptible to mosquito saliva98. The high concentration of saliva due to repeated exposure to mosquito create an immunosuppressed environment, whereas decreasing saliva exposure and concentration increase T helper cells response97. Also,in malaria-endemic area there exists some demonstrable evidence suggesting that the acquisition of anti-Plasmodium response differs in children with varying exposure to a mosquito bite with a high level of cytophilic antibody response to Plasmodium97.
Humans have shown some elastic immune response to various toxic infectious, nutritional and microbial exposures encountered throughout life, this coupled with environmental exposure drives epigenetic modifications which allow innate immune cell programming99. It is now evident that such epigenetic programming and modifications are important in the immune system adaptation, activating the T cells and memory response99,100. These memory responses are produced during Plasmodium infection thus, minor changes in the environmental temperature that enhance acetylation or deacylation processes may lead to diverse responses among individuals101,102, affecting expression or the success of the immune response directed against the parasite. Others may affect the distribution of resources within the mosquito and affect the survival of plasmodium development. Environmental factors regulating the anti-Plasmodium immune response include nutritional status, temperature, population density and parasite frequency and strains.
Effect of nutrition on anti-Plasmodium immune response: Malnutrition and malaria are recurring public health problems in sSA103. In the, Gado-Badzéré region of Eastern Cameroon an association was made between child malnutrition and specific anti-Plasmodium falciparum immune responses; particularly malnutrition impact on the status of iron and on the total anti-P. falciparum IgG levels in children living in the Gado-Badzéré refugee camp, in Eastern Cameroon, this study confirmed that nutritional status and serum iron levels had no significant influence on children’s anti-Pf IgG T levels103.
Low level of protein intake could affect the generation of robust response to anti-malarial vaccination, a fact from clinical trials on malaria vaccine candidates in elite countries against endemic countries104. Protein consumption among individuals residing in most endemic countries may vary depending on the region of residence and financial status105. Reductions in dietary protein consumption over the period of immunization result in a drop in immunization results and a drop in the frequency of circulating CD4+ T cells and hepatic NK cells105, leading to an imbalance in the amount and quality of Hb produced and released into circulation (a classical condition known as hemoglobinopathies). In mice fed with high-protein food, the dietary protein was shown to have increased the T cell-independent secretory IgA-induced cytokine production with higher amounts of extracellular vesicles106. Microbiota present in the gut has been shown to have capacity to balance the risk of infection, transmission and severity of malaria. Some recent studies indicated that the gut microbiota could shape the host immune response, including such response to the different immune-modulatory drugs such as those targeting the immune checkpoint inhibitors, anti-proliferative drugs and inflammatory cytokine inhibitors107. Consequently, the gut microbiota is a key factor contributing to the overall response of the host metabolism and immune response108.
Despite overarching health challenges associated with these hematological disorders, it provides some important strategies in the management and control of malaria episodes especially during the attack of the human red blood cell by the merozoites55,109. Merozoite’s invasion of the RBCs and subsequent hemoglobin breakdown are impaired by interactions involving the formation of sickle-cell and thalassemia, a reaction which is thought to prevent merozoite multiplication and further invasion56.
Temperature effect on anti-Plasmodium immune response: The rates of both humoral and cellular immune responses in a major malaria vector can be influence by temperature of the mellieu, especially during the lysis of oocyst110. However, following quality laboratory approach of traversing the function of the immune-system and mosquito pathogenic interactions using a narrow temperature, has not provided the required optimum range of parasitic multiplication in the vector110. Knowing this range may be important as the temperature has shown to have a significant influence on the expression of defensins, a cysteine-rich peptide known to possess antimicrobial effects and serve as host defense peptides111. At a temperature of approximately 26°C, mosquitoes experience an increased level of defensins expression within the first 6-12 hrs, bringing about the reduction of the host-parasite level, regulating their immune response110. Moreover, the temperature is thought to also influence the time span of the latency period and its recovery time because it regulates the resistance mechanism by affecting the parasite growth, which is independent of the mosquito and the host110,112. Temperature also meaningfully affected the expression of cecropin-1 (CEC1), another important peptide that helps in cell defense against foreign molecules. This occurs in a manner that is dependent on the kind of the immune challenge113.
Population effect on anti-Plasmodium immune response: Malaria naïve individuals infected by a mosquito have a likelihood of developing clinically immunity upon recovery from the infection, however, such maybe shortlived unless the individual is re-infected114. This is suggestive that such clinical immunity can only be sustained in the course of high infection and its often lost in the course of low infection115. In exploring this concept, a comparative study of the life-cycle transmission effectiveness of naïve and clinically immune respondents, using subgroup reproductive numbers showed a possible elimination of the parasite116. Simply, these kind of analyses are usually defined as; average number of secondary infections from a single infectious individual in an otherwise totally susceptible population. Whilst, the reproductive number for naïve cases (RNN), is defined as; the average number of secondary infections generated by a single naïvely infectious individual in an otherwise totally naïvely susceptible population117.
Parasite effect on anti-Plasmodium immune response: Helminthic infection is known to modulate the response against Plasmodium parasite in the immune environment generated being that persistent helminth infection in its host significantly modifies the host’s vulnerability or the defense to such host from Plasmodium118. The genetic make-up of the host, the form of helminth and the duration of the initial helminthic infection, for the resultant immune response to Plasmodium are paramount for such modifications119. The impact of helminth-Plasmodium co-infection on helminthic infection synergizes or increases with the T helper 1-type immune response. Such co-infection may be successful in prompting a response that impede Plasmodium replication but increases the pathology and impermanence in the host, suggesting a possible pathway in the control of malaria120. Repeatedly, helminth-infected mice presented a move towards T helper 2-type immune responses, causing the host to be more susceptible to Plasmodium infection, approbating their replication and having some protective on the host against severe malaria121. It can therefore be inferred that malaria immunity is influenced by helminth infections.
Co-infections interactions between Plasmodium infection and other unrelated pathogens: Malaria, a prevalent vector-borne disease poses a global health challenge as over 405,000 malaria-related deaths were recorded globally in 2019122. The propagation of malaria can be corresponded to the complicated nature of infection and the numerous genetically different parasites observed within a bout of infection. Genetically different malaria parasites can infect an individual through two basic paths, i.e., an individual may be inoculated by two or more infected mosquitoes, that bear a distintive parasite genotype or a single mosquito habouring greater than a single parasite genotype inoculates a single individual, leading to a condition called malaria co-infection123.
Co-infection of Plasmodium with Ebola: Across sSA malaria is highly prevalent thus, deductively, the prevalence of viral-Plasmodium species and co-infection may dictate responses to viral infection. The sporadic outbreak of Ebola disease occurs in many African countries where there exists a high incidence of malaria124. Interestingly, Ebola and Plasmodium have been found to link in several ways that can be of public health importance assuming Ebola virus disease-with its related anguish or impermanence are reduced or decreased following such interactions124. Severe Plasmodium infection is associated with the activation of pro-inflammatory pathways, which has been connected in influencing response to other parallel infectious agent e.g., respiratory viruses, enteric bacteria and Human Immunodeficiency Virus (HIV) disease124. Infection by Plasmodium parasite, was seen to drop viral blood level and associated diseases of chikungunya virus, a more acute RNA virus warranted through proinflammatory responses via Interferon-Gamma (IFN-γ) production125. Available evidence suggests that bacteria co-infection may result in severe malaria outcomes by exacerbating the inflammatory response that characterizes malaria126,127. Several lines of proof point to co-infection with non-typhoid salmonella species and other Gram-negative bacteria, common in developing countries as major contributors to malaria-related morbidity among African children128. Malaria can raise the susceptibility to concurrent bacteremia and the stimulation of neutrophils dysfunction thus causing a drop in chemotaxis and oxidative burst129. Malaria infection predisposes individuals to concurrent infections and such individual responses to inflammatory stimuli of bacterial origin127,130.
Co-infection of Plasmodium with Human Immunodeficiency Virus (HIV): Malaria and HIV, both are public health concerns131. The considerable epidemiological overlap between these two dissimilar diseases may promote a reasonable number of co-infections131. Albeit, evidence on HIV-P. vivax co-infection (HIV/PvCo) is uncommon, as likened to the available knowledge related to P. falciparum on the African continent132. Moreover, it remains unclear if HIV infection may change the clinical course of malaria disease associated with the P. vivax variant and resultantly raise the risk of complications132,133.
Available evidence suggests malaria and HIV infections may undergo bidirectional and synergistic interaction, producing an exponential increase in their lethal presentation134. The HIV impairs immune responses to malaria parasites, resulting in an inability for parasitic check and removal, leading to increased parasitic loads, which in turn, hightens malaria transmission rates131,135. Clinically, HIV is related to a raised incidence in P. falciparum malaria132, including the severe kind, characterized by anemia, cerebral malaria and higher risk of congenital infections132,136,137. From clinical evidence, it seems the impact of HIV on the malaria disease severity is restricted to patients with CD4+ T cell counts of less than 350 cells μL–1 132. This is following that malaria infections are associated with strong CD4+ T cell activation and raised parameters of pro-inflammatory cytokines131, which worsen the clinical manifestation, hightens HIV progression to AIDS with the maintenance of the viral load131,137. The immunosuppression caused by HIV infection may decline the control of malaria. Similarly, HIV treatment can deter malaria therapy, with sufficient rise in adverse effects, including a potential selection of therapy resistant plasmodium132,138. Parasite co-infection has been indicated to raise HIV viral load and transiently drop CD4+ T cell count132,133, albeit such interactions are mostly described for P. falciparum132. In all, it was observed that acute malaria is connected to raised HIV loads139 with serious drop in CD4 cell count22,139, worsening the opportunistic bacterial, viral and protozoan co-infection. However, more findings may be required in elucidating and determining the pathology and the clinical outcomes of HIV-malaria co-infections140.
Co-infection of Plasmodium with helminths: The infection of tissue invasive helminths is a good example of an immuno-regulatory environment that is influenced by IL-10 and TGF-beta cells, modulating the repression of T cell proliferation with decreasing of IL-2 and IFN-gamma outcome, all in response to related/unrelated antigens126. Using filarial nematodes as a tool, the effects of helminth infection on anti-malaria immunity was characterized in more detail141. Moreover, experimental models of co-infection with filarial worms and plasmodia in rodents show a protective effect against acute malaria142. The outcome of observations in human and research carried out using malaria models discussed above, are largely determined by the rate of inflammation, which is evoked by infection. Different levels of parasitic severity and co-infection with unconnected pathogens are key factors in determining the inflammation balance and regulatory immune response priming either hightened malaria-related mobidity or some degree of protection against infection126.
Consequences of malaria on the host global immunity and on concomitant diseases: Different mechanisms have been developed by various parasites, for the evasion or manipulation of the immune response and other establish infection7. Using experimental animals espercially rodents (housed and maintained to a high microbiological status), in vivo studies had been carried out to investigate these host-parasite interactions143. Practically, it is increasingly becoming obvious that pathogen co-infections within the same host are generally seen in most areas of the world143. For instance, prolong infection with pathogens such as malarial parasites, Mycobacterium tuberculosis, soil-transmitted helminths and HIV may affect one-third of the human population of some developing countries143. These infections seems in most cases to be associated with the development of systemic and mucosal CD4+ T helper cell type 2 (Th2) polarized immune responses144 and are marked by an raised number of cytokines like; Interleukin-4 (IL-4), Interleukin-13 (IL-13), eosinophilia, production of immunoglobulin E (IgE) and stimulation of alternatively activated (M2) macrophages and type 2 innate lymphoid cells (ILC2)145.
The features of immune response as a consequence of infection by parasites of different origin can greatly differ from each other. For instance, trypanosomes and plasmodium are both vector-borne of unicellular protozoan parasites origin and are causative agents for trypanosomiasis and malaria, in both humans and animals. These parasite species have potential to develop persistent infections in the host’s bloodstream: Bearing, the trypanosomes live extracellularly, with ability to initiate a cyclical rounds of intracellular infection within erythrocytes, forebear of the RBC. Responses to infection with these protozoan parasites by the host immune system are markedly different from the predominantly Th2- polarized reactions that are triggered by helminth infections146. Enhanced levels of pro-inflammatory cytokines like the Interferon-γ (IFNγ), Interleukin-12 (IL-12), increased levels of CD4+ Th1 cells, CD8+ T cells and NK cells and the inducement of pro-inflammatory classically activated macrophages (M1), are associated with malaria infection,. Moreover, several lines of evidence indicated that modifications to these pro inflamatory responses may be due to co-infection with other pathogens, with consequencial effects on disease susceptibility147. Several inflammatory cytokines such as IFN-γ, IL-1, IL-17, TNF and IL-4 can upregulate Polymeric Immunoglobulin Receptors (pIgR) following the signaling of TLR4/NFκB148.
These alternatively activated macrophages play some key roles in repairing the tissue damage, consequence of helminth infection. It is evident that co-infection with these parasites may change the vulnerability to other important pathogens and/or influence vaccine efficacy via effects on host immune reactions. Co-infection with some pathogens can also hinder accuracy for disease diagnosis143. It is important to understand the various processes associated with immune system responces to infection with different types of parasites and the consequences underpinning the vulnerability to infection with other pathogenic microorganisms. This understanding will help in disease diagnosis and design of novel vaccines or therapeutics to more effectively control the spread of infectious diseases in man143. The effects of Plasmodium infection on the host global immunity and related diseases explained in this review include the effects of malaria on helminth co-infection; effects of malaria on schistosome (blood fluke) infections; effects of malaria on African trypanosome; effects of malaria on bacterial infections such as Salmonella pathogenesis; effects of malaria on a viral infection like Human Immunodeficiency Virus (HIV).
Effects of malaria on helminth co-infection and its consequences on host global immunity: In malaria-endemic regions (sSA and Asia), sufferers are often co-infected with soil-transmitted helminths. The sequel, the larval phases of certain parasitic helminth species do wander to the host’s lungs to illicit pathological activities143. Typically, the larvae set into action, Th2 immune responses, causing the activation of other related primed macrophages. Such combined actions of these epigenetics are implicated in the coordination of parasitic clearance and the repair of host worn-out tissues during phagocytosis149. In BALB/c mice infected with hookworm parasites such mechanism was demonstrated using e.g., Nippostrongylus brasiliensis, such that the development of a Th2-polarized immune response to the hookworm infection was impaired in mice group co-infected with P. chabaudi malaria parasites150, resulting in the lower expression of other related activated macrophage-derived factors in the lungs like chitinase151. These experimental study including, incidence of lung granulomas induced by infection with Litomosoides sigmodontis microfilariae, was reduced in mice, which were co-infected with either P. chabaudi or P. yoelii malaria parasites152. Cumulatively, these results suggests that malaria infection could negatively impact the host's ability to induce a Th2-polarized specific immune response due to co-infection by helminths150. Further research will be needed in this regard to fully validate the processes of how malaria infection may modulate these effects. Undoubtedly, the understanding of the various pathways including, lipid peroxidation, fatty acid and protein syntheses, glycoprotein activation153, the regulation of reactive oxygen species (ROS) generation in the red blood cell, etc., known to be associated with both human and vector infections will be very important in an attempt to fully situate if some of these actions are a direct result of the malaria parasites or are an indirect outcome of a strong combination of Th1-polarized immune response/cytokine reacting to the malaria infection among individuals.
Effects of malaria co-infection on schistosome (blood fluke) infections and its consequences on host global immunity: Schistosome infection causes chronic inflammatory disease in humans and animals154, leading to the evolution of some acute pathological conditions, notable anguish and economic loss. Schistosome is a snail vector disease, transmitted via the skin upon contact with polluted water inhabited by the snail. Like Plasmodium parasites, schistosomes establish prolong infections in the mammalian host’s blood attaining maturity, mate and produce considerable numbers of eggs. These eggs permeate into the intestines and bladder where they are excreted via feces and urine. The eggs are in turn deposited in the host tissues resulting in prolong inflammation, tissue distruction and fibrosis154. Schistosomes typically induce a Th2- polarized immune response in the mammalian host, enabling the establishment of prolong infections, persisting for years in the host143,155.
Targeting the blood stage of this infection may serve as a valid candidate for the establishment or development of treatment protocols using secondary metabolite of plants origin with the potential for truncating schistosome transmission. Along this line in previous studies, it was established that the transmission of schistosome infections coupled with the degradation of the host hemoglobin is enhanced by hemoglobinase, cysteine and elastase-like serine proteases156, enzymes that are known to be utilized by Plasmodium parasites during its erythrocytic life cycle5,157,158. Plants belonging to the family of Apocynaceae such as Calotropis procera (Sodom apple) and marine bacterial cells such as Actinobacterium cells are known to possess inhibitors with the potential of halting the catalytic activities of these proteases, suggesting a possible control mechanism for schistosome infection5,157,158.
In research using laboratory animals such as mice, it was demonstrated that gastrointestinal nematode infection can influence the pathogenesis of subsequent schistosome infection143. Within the large intestine a chronic Trichuris muris infection can be enhanced by the survival and migration of S. mansoni to the portal system. Thus, the disease burdens associated with schistosome worm and egg genetics were enhanced when compared to mice infected with S. mansoni alone159. This finding suggests that the immuno-regulatory mechanisms or effects,elicited in the mucosa of the large intestine allowing the gastrointestinal helminth to establish chronic infection may or could be extended to other host systems159. This may increase the exposure of host susceptibility to subsequent co-infection with other helminth parasites. With a longitudinal study, a similar effect has been reported to occur in a naturally-affected livestock species160, involving free-ranging African buffalo infected with Cooperia fuelleborni, had greater burdens of schistosomes (Schistosoma mattheei) than those with negative presence of this nematode species. However, the processes that moderate such effects in the co-infected animals remain unknown, albeit the variations of the host in susceptibility to gastrointestinal nematodes161.
Effects of malaria co-infection on African Trypanosome and Trypanosome cruzi and their consequences on host global immunity
African Trypanosome: Hemoflagellate protozoan parasite is a single cell and are transmitted between mammalian hosts through genus Glossina tsetse flies that are blood-feeding162. The Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense subspecies are known to cause a disease, the human African trypanosomiasis, in endemic regions within the tsetse fly belt across sSA143. The protozoan strains T. congolense, T. vivax and T. Brucei in animals, causes African trypanosomiasis with serious and substantial economic strains on the African livestock industry162. During the parasitic life cycle within the mammalian host, the disease-causing potential of this protozoan is established and are thought to be instituted by the intradermal inoculation of metacyclic trypomastigotes by the vector143, with the extracellular parasites reaching the draining lymph nodes, via the afferent lymphatics and spreads to the general system163,164. During this course, the metacyclic trypanosome forms metamophose into long slender bloodstream forms, enabling their survival within the mammalian host143. Infecting C57BL/6 mice with T. brucei, leads to an initial parasitemic wave, with cocomitant and enhanced expression of IFNγ by the host, due to attempts by the host to control the infection. However, due to IFNg over expression in the host’s bloodstream the trypanosomes are thought to also cause notable immunosuppression, promoting the establishment of prolong infections in the hostile environment143.
Malaria severity and trypanosomiasis was found to be elevated in mice that were co-infected with P. berghei and T. brucei, with resultant drop in the rate of survival with higher parasitaemias level, leading to more severe anemia and hypoglycemia165. Evidently, the resultants effects of each of these infections are strong pro-inflammatory response leading to the over expression of IFNγ. These studies so enumerated makes, further studies necessary so as to situate the additive/synergistic consequences of each infection on IFNγ expression and their corollary for the increased disease severity observed in the co-infected mice.
Ulcerations as a consequence of trypanosomes infection may mediate host vulnerability to infection with other pathogenic bacteria. In line with these thoughts, using chronically infected mice, with either Brucella melitensis, B. abortus or B. suis, the load of bacteria in the spleen was found to decreased considering if the same were also co-infected with T. brucei166. This findings suggests that in the absence of functional IL-12p35/IFNγ signaling, the effects of T. brucei infection on Brucella loads in co-infected mice may have been impaired, again leading credence to the earlier posture that the strong pro-inflammatory IFNγ-mediated immune response induced by the T. brucei infection may have aided the elimination of Brucella. Thus, it is most propable that, infections with T. brucei may cause reasonable levels of immunosuppression and immunopathology, whilst under some circumstances, the host’s response to T. brucei infection can improve defence against co-infection with other pathogens166.
Consequences of malaria infection on Salmonella pathogenesis and its effects on host’s global immunity: “Salmonella” is a Gram-negative bacterium and can be acquired from polluted food such as milk, meat and eggs. They are a common cause of diarrhea in children and adults. The co-infections of this bacteria with other parasites, like, helminths and Plasmodia, have been reported to increase vulnerability and or pathogenesis of, salmonellosis143.
Previous discussions on the association explained the connection, induction of a Th2-polarized immune response to helminth infection with the concomitant decreased development of Th1-polarized immunity to co-infection with other pathogens167. However, the co-infection of mice with the gastrointestinal helminth H. polygyrus has also been shown to increase the infection pathogenesis with Salmonella enterica serovar Typhimurium, an outcome that are seemingly independent of the actions of Th2 cells and regulatory T cells168. Similarly, Heligmosomoides polygyrus co-infection was indicated to disrupt the metabolic profile of the small intestine, affecting the invasive capacity of S. typhimurium as a consequence169. The helminth infection has also been implicated in mediating such effects through the enhancement of the genes for bacterial expression of Salmonella Pathogenicity Island 1 (SPI-1)168. These findings are all suggestive of a possible novel immune system through, which a helminth-modified metabolome in the host’s intestine may become promote susceptible to bacterial co-infection.
The presence of non-typhoid Salmonella (NTS), its association with co-infection, with resultants high malaria mortality, coupled with a study of hospitalized children in North-Eastern Tanzania indicated that a decline in malaria cases may share similar associated decline in NTS and other forms of bacteremia incidence170-172.
The turnover rate of erythrocyte disintegration and hemolysis, with resultant anemia, usually occur due to the attendance infection of malaria parasites within the RBCs. Cases of patients with severe malarial anemia has shown alliance between NTS infection with hemolysis143. With hemolysis large amounts of cell-free heme that are harmful to the host are released. Thus to cushion this effect, Heme Oxygenase-1 (HO-1) expression is prompted to breakdown the heme to provide tolerance toward some of the pathological effects of malaria171. This actions of HO-1 also provide cytoprotective role and hence, limite the production of ROS173. Moreover, results emanating from recent research174, show (a) Analysis of neutrophils from malaria-infected children174 and (b) Indicates that the actions of HO-1 in granulocytes in response to hemolysis during malaria infection impairs their oxidative burst activity and production of reactive oxygen species, leading to dysfunctional granulocyte mobilization and long-term neutrophil dysfunction. Furtherance, Salmonella is able to survive, proliferate within neutrophils during malaria infection due to their decreased oxidative burst activity, with increased NTS susceptibility174. These findings are an indication of how a host-induced cytoprotective response to one of the pathological consequences of malaria infection (hemolysis) can significantly impair neutrophil-mediated resistance to co-infection with other pathogens.
Effects of malaria and viral infection, its consequences on host’s global immunity: The initiation of a Th2-polarized immune reactions to the parasite infections seems to impair the development of effective antiviral immunity, in cases of parasitic co-infections. Using mice co-infected with the gastrointestinal helminths, Trichinella spiralis or H. polygyrus (exposed to P. berghei) and mouse norovirus (MNV), the validity of such thoughts was demonstrated175. The results of the findings, reported an increased viral loads and reduced levels of virus-specific CD4+ T cells expressing IFNγ and TNF-α in comparison to mice infected with only norovirus175. The Th2 cytokines production during infection caused by both helminth and P. berghei are connected with the expression of the signal transducer and activator of transcription-6 (STAT6), via actions of differentially activated macrophages167. Moreover, evidence from STAT6 null mice, shows viral loads were decreased when compared to wild-type controls implying that the activation of STAT6-dependent activated macrophages during both infections can impair the initiation of antiviral innate and adaptive immunity176. Furthermore, the expression of IL-4 and activation of the transcription factor STAT6 during infection can promote the regeneration of latent γ-herpes virus infection, as well as impairing the efficacy of anti-viral immunity176.
Human Immunodeficiency Virus (HIV): Evidently, hygiene have been implicated in co-infection prevalence. For insatnce, the study on HIV-infected Ugandans177 indicated a high occurrence of parasitic infections (especially Necator americanus) and co-infection with hookworms, which corresponded with reduced peripheral blood CD4+ T cell levels than those, infected with only HIV. This study, is corroborated with the proposition that individuals, co-infected with hookworms and HIV are at a distinct immunologic disadvantage when compared to those infected with HIV alone. Using a helminth/retrovirus co-infection model in mice178, the hypothesis was further tested experimentally. The level of virus-specific CD8+ T cells, FoxP3+ regulatory T cells and cytokines were similar in co-infected mice and those infected with only the more receptive virus. The raised viral loads in co-infected mice were rather associated with decreased titers of neutralizing virus-specific IgG2b and IgG2c antibodies177. However, earlier studies reported no beneficial effect of antihelminthic treatment on HIV viral loads [plasma HIV-1 RNA concentrations143 and other studies have suggested that helminth co-infections do not increase HIV infection177. On the other hand, a similar reaction may occur in malaria and HIV co-infections, owing to the fact that malaria infection has been suggested in earlier reports to possess immunoregulatory effects on CD4+ T cells and CD8+ T cells7. This may probably increase the risk of HIV infection because of the effects of increased viral loads upon the release of cytokines. Although, more research to ascertain malaria co-infection with HIV using a mice model to establish the level of impaired host global immunity as a result of such co-infections is needed.
Future perspectives: The immune regulation of infection resulting from Plasmodium parasites involves mostly CD4+ and CD8+ lymphocytes, antibodies and cytokines including gamma-interferon24. This, however, has been shown to be impaired by many factors such as the complicated life cycle of Plasmodium179, antigen variation, the emergence of Plasmodium resistance to most common and affordable anti-plasmodial chemotherapy and the adjustment of immune feedback by the pathogen and/or environmental factors24,180. The parasites have different mechanisms for evading both the vector and the human host immune feedback7. The immune-evading process in mosquitoes depends mainly on the Pfs47 gene that inhibits Janus Kinase (Jk) -moderated activation, while the host complement factor also protects human complement immune attack of extracellular gametes in female Anopheles mosquito midgut, suggesting a possible pathway to be studied in the development of a novel approach for the check of malaria-blocking the transmission of Plasmodium7,181.
An important biological receptor, ferriprotoporphyrin ix (FP, a product of hemoglobin degradation, via, the action of chloroquine), identified as a high-affinity drug-receptor of malaria parasites (with an appropriate affinity for chloroquine), having a dissociation constant (kD), on the order of 10–8 M, with specificity for amodiaquine, quinacrine, quinine and mefloquine, is believed of having a link with the immune response to malarial disease treatment among patients182. This is evidenced by the observation that FP and its combination with chloroquine reduce the ability of cell membranes to maintain cation gradients, with a capacity to lyse normal erythrocytes, Plasmodium berghei (animal model Plasmodium parasite) and P. falciparum. Therefore, chloroquine and related drugs, act as antimalarial agents by shifting FP away from natural haem binders and into toxic drug-FP complexes182. Also, FP exuded from hemoglobin, either spontaneously or by oxidant drugs, might add to hemolysis and defence against malaria in patients with Heinz body hemolytic anemias183.
Another promising research revealed the ability of chloroquine to bind tightly to FP, diverting it away from the soluble detoxifying substance in malaria parasites and subsequently delay its isolation into malarial pigment182. Malaria parasites upon exposure to chloroquine while degrading hemoglobin accumulate a chloroquine-FP complex, toxic enough to kill the parasite183. It seems FP has a detergent-like effects on biological membranes, which may account for its lytic activity thereby enhancing immune response to malarial disease.
Plasmodium undergo a necessary and asymptomatic developmental phase within the mosquito midgut184 and the host’s liver before infecting the epithelial cells lining the mosquito midgut and the red blood cells to cause infection in both the vector and human185. The parasites hijack and infest critical pathways during the host and vector infections. It is critical to control this cycle of events because of the complex biological cycle of Plasmodium species, antigen variation, the high frequency in the reoccurrence of parasite resistance to most common and affordable anti-plasmodial chemotherapy, the regulation of immune feedback by the pathogen and/or environmental factors and the development of parasite variants4. Both host and vector respond to the parasite evasion, resulting in the release of antigens which aids the regulation of immune responses185. Different research has linked the infectiveness of P. falciparum and the capacity of the parasite to evade the human and vector immune system by different mechanisms as being responsible for its immune response6. Plasmodium falciparum and other malaria parasites were reported to have different mechanisms for evading both the vector and the human host immune responses7.
The mechanism of immune-evation by mosquitoes depends mainly on the Pfs47 gene that hinders Janus kinase-modulated activation, while the host complement factor also protects the human complement immune attack of extracellular gametes in female Anopheles mosquito midgut7. This suggests a possible pathway to study in the development of a novel approach for the check of malaria181, by blocking the transmission of Plasmodium. The Plasmodium transmission both in the vector and the host is facilitated by TRAP of sporozoite, with an adhesive domain (‘A-domain’)4. Anopheles saglin protein recognizes the sporozoite-TRAP domain resulting in the transmission of the parasite, which possibly brings about an immune response4. The structure of the sporozoite-TRAP was revealed to possess a special motif for the binding of cyclic cysteine peptides like SM1 peptide and cyclotide antimicrobial peptide4. The SM1 peptide and cyclotide antimicrobial peptide anchor into the putative region of the Anopheles saglin protein, thereby preventing the sporozoite-TRAP attachment and hence abrogating the pathway leading to Plasmodium transmission4. It is, therefore, important to establish the possibility of anti-Plasmodium IgG, SM1 peptide and cyclotide antimicrobial peptide stimulating the Pfs47 gene, which in turn inhibits the Janus kinase-mediated activation, hence protecting the human complement immune attack. The interaction between these complement factors may bring about an immune response to Plasmodium infection and a novel control mechanism for malaria.
It is also worthwhile to consider a promising approach that involves an understanding of different genetic factors within the vector and the human ‘druggable’ genome186, that are critical to Plasmodium infection in midgut epithelia and hepatoma cells. This would provide laudable measure in the check of malaria. The control of Plasmodium infection by immune response usually involved CD4+ and CD8+ lymphocytes, antibodies and cytokines like gamma-interferon, which are impaired by many parasite factors previously mentioned24. The process of antibody-modulated parasite inhibition is important in understanding the function of antibodies against different targets of antigen in the immune response by vaccination and establishing protection187. Targeting the circumsporozoite protein, a major protein that crosses the gap between endothelial cells and Kupfer cells is important in regulating the immune feedback to Plasmodium parasitaemia7,24,187. This, in turn, upregulate the inflammatory T helper Th2 cytokines and increases the expression of major histocompatibility complex (MHC-1), thereby increasing T cell activity. However, regulation or manipulation of the T-regulating cells after malaria infection may be paramount in eliminating the parasite and conferring immunity against infection. The T regulatory cells are activated by Cytotoxic T Lymphocyte-Associated Protein 4 (CTLA-4), that hampers immune response1. A novel approach that offers blocking of CTLA-4 during the phase of CD4+ cell expansion tends to eliminate the parasite. The CTLA-4 therapy may be necessary for protecting against future infections. Functional analyses of the novel P. vivax-specific host factors in the future, should be directed towards the development of transmission-blocking vaccines and enhance the generation of new intervention techniques or modify current ones.
Many human genetic factors have been linked with natural resistance to malaria parasites. These include the Dantu polymorphism, which is a strong protective variant in RBC glycophorins, a RBC membrane proteins, they are mostly present in people living on the Kenyan Coast43. Other human genetic factors like IgG immunoglobulin, blood group O+, alpha thalassemia, glucose-6-phosphate dehydrogenase mutated genes, hemoglobinopathies, Human Leukocyte Antigens (HLA), ethnicity, FcγR gene polymorphisms and mutation at glycophorin gene cluster resulting in the presence of two GYPB-A hybrid genes, encoding the Dantu blood group variant has gained attention in research involving the regulation of anti-Plasmodium immune responses. It is therefore paramount to establish an association between these human genetic factors in ensuring natural resistance to Plasmodium parasites. The introduction of different genetic techniques such as gene shredding or silencing and frameshift mutation to bring about a similar phenomenon like those reported by Portugal et al.43 may produce functional genes with enhanced resistance to malaria parasites. Gene silencing techniques used to reduce the levels of FLVCR (a Plasmodium cell surface transport protein, Feline leukemia virus subgroup C receptor, which pumps heme out of the cell) in the mosquito midgut may produce similar effects in regulating genes responsible for human druggable genome4. For example, mutations and deletions of two similar genes on chromosome 16 (that code for alpha chain) and a single gene on chromosome 11 (that code for beta chain)55,188 in hemoglobinopathies may serve as a candidate for the novel control of malaria episode, especially during the attack of the human red blood cell by the merozoites.
It will be worth investigating the role of dietary intervention in boosting immunity in relation to the gut microbiota when infected by Plasmodium parasites. The interplay of gene-diet interactions (Nutrigenomics) is an emerging approach in nutritional research. A recent study conducted on Ugandan children demonstrated that gut microbiota, specifically bacteria, can mediate the adversity of malaria by regulating the spleen germinal center189. Villarino and co-workers revealed that specific members of bacteria (Lacto bacillus and Bifido bacterium) of the gut microbiota were responsible for the regulation of malaria adversity in mice. However, the effect of malaria on the structure of the gut microbiota is not well understood, thus maybe confusing. Mooney and co-investigators showed that the gut microbiota decreases resistance to intestinal colonization of non-typhoidal Salmonella (NTS) and malarial immune effects may encourage vulnerability to disseminated NTS infections190. But, Mandal and colleagues in a study that involved one hundred Kenyan infants showed that Plasmodium infections and antimalarial treatment with artemether-lumefantrine had very minimal effect on human gut microbiota189. These confusing findings demand for futher research that will help elucidate these gene-diet interactions and immunity to malaria.
There are many pathways at the molecular levels, regulating phenotypes that showcase the effectiveness of host immunity, notably parasitemia and IgG levels, that are important given the current lack of an effective vaccine and the need for new management options72. The knowledge of the genetic basis of parasitemia levels and IgG levels through crucial examples including the hemoglobinopathies are very important, as such demonstrated that, the protective effect of HBB variants on malaria clinical phenotypes, may partially be modulated through parasitemia and cytophilic IgG levels72. Another promising example is the IgG receptor FcγRIIa, encoded by FCGR2A, such that H131 homozygotes displayed higher IgG2 levels and were protective against high parasitemia and onset of malaria symptoms. Figure 3 presented that, P. falciparum infection leading to parasitemia, clinical attack and severe malaria via IgG and IgG2-mediated pathways are enhanced by merozoite invasion of the RBC (utilizing important proteins such as cysteine, serine and aspartate proteases), merozoites multiplication in the RBC and subsequent degradation of hemoglobin. Targeting these proteases using natural peptides or protease inhibitors will provide an important path to follow in the design of novel antiplasmodial chemotherapy. The presence of Plasmodium serine and cysteine protease inhibitors in marine actinobacterium cells and Calotropis procera latex, respectively, were reported with potential antiplasmodial activities5,157,158. It is important, therefore, to establish the genomic and metabolomic sequences of these natural proteases with inhibitory potential against the Plasmodium parasite. This will provide further data on the sequence of interactions between these protease inhibitors and the Plasmodium parasite proteins in halting malaria.
CONCLUSION
Nigeria is one of the major countries suffering form malaria, within the Sub-Saharan African nations and the World in general. Malaria as a global disease require urgent and needed attention, preventing it from falling into the orphan disease category. Therefore, the needs for much attention to research involving public awareness creation, epidemiological survey, treatment protocols that will includes the management of this disease using secondary metabolites from medicinal plants that may alter some known proteins/epigenetics markers is now and this cannot be overemphasized.
Hence, concerted scientific efforts are required moving forward to harnessing molecular and biophysical tools to explore some of these biomarkers (espoused in our review) as a means to disrupt malaria propagation and transmission by the different parasitic variants.
In this regards, the current molecular understanding of epigenetic processes will help scientists to keep pace to unravel the remarkable capabilities of malaria parasites to survive in a myriad of changingand often hostile environments.
SIGNIFICANCE STATEMENT
Malaria is of global concerns with threat to public health leading to economic under developments. It is estimated that, in Sub-Sahara Africa one to three million children die of malaria each year. This is an alarming statistics hence; concerted efforts are required to reduce and eliminate this scourge,. This goal, remain elusive as efforts to eradicate malaria have yielded little results with parasitic resistance increase to most commonly used drugs. This review is carried out to further espouse the molecular and epigenetics factors propagating the disease or conferring parasite resistance. This will enable further understanding of the disease, by enhancing development of effective and efficient novel drugs from secondary metabolites of plant origin, which may alter some proteins/epigenetic markers.
REFERENCES
- Phillips, M.A., J.N. Burrows, C. Manyando, R.H. van Huijsduijnen, W.C. van Voorhis and T.N.C. Wells, 2017. Malaria. Nat. Rev. Dis. Primers, 3.
- Srivastava, A., P. Srivastava, A. Pandey, V.K. Khanna and A.B. Pant, 2019. Phytomedicine: A Potential Alternative Medicine in Controlling Neurological Disorders. In: New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads, Khan, M.S.A., I. Ahmad and D. Chattopadhyay (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780128146194, pp: 625-655.
- Okoh, M.P., R.K. Singla, C. Madu, O. Soremekun, J. Adejoh, L.A. Alli and B. Shen, 2021. Phytomedicine in disease management: In-silico analysis of the binding affinity of artesunate and azadirachtin for malaria treatment. Front. Pharmacol., 12.
- Adejoh, J., M.O. Egua and M.P. Okoh, 2018. Control of malaria by blocking transmission of plasmodium. Int. J. Biol., 10: 29-41.
- Adejoh, J., B.A. Inyang, M.O. Egua, K.C. Nwachukwu, L.A. Alli and M.P. Okoh, 2021. In-vivo anti-plasmodial activity of phosphate buffer extract of Calotropis procera latex in mice infected with Plasmodium berghei. J. Ethnopharma., 277.
- WHO, 2016. World Malaria Report 2015. World Health Organization, Geneva, Switzerland, ISBN-13: 9789241565158, Pages: 246.
- Belachew, E.B., 2018. Immune response and evasion mechanisms of Plasmodium falciparum parasites. J. Immunol. Res., 2018.
- Mandala, W.L., V. Harawa, F. Dzinjalamala and D. Tembo, 2021. The role of different components of the immune system against Plasmodium falciparum malaria: Possible contribution towards malaria vaccine development. Mol. Biochem. Parasitol., 246.
- Lombardo, F. and G.K. Christophides, 2016. Novel factors of Anopheles gambiae haemocyte immune response to Plasmodium berghei infection. Parasites Vectors, 9.
- Ghosh, A.K., I. Coppens, H. Gårdsvoll, M. Ploug and M. Jacobs-Lorena, 2011. Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc. Natl. Acad. Sci. U.S.A., 108: 17153-17158.
- Crompton, P.D., J. Moebius, S. Portugal, M. Waisberg and G. Hart et al., 2014. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol., 32: 157-187.
- Smith, R.C., J. Vega-Rodríguez and M. Jacobs-Lorena, 2014. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Memórias Inst. Oswaldo Cruz, 109: 644-661.
- Clayton, A.M., Y. Dong and G. Dimopoulos, 2014. The Anopheles innate immune system in the defense against malaria infection. J. Innate Immun., 6: 169-181.
- Dong, Y., S. Das, C. Cirimotich, J.A. Souza-Neto, K.J. McLean and G. Dimopoulos, 2011. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog., 7.
- Sinnis, P. and F. Zavala, 2012. The skin: Where malaria infection and the host immune response begin. Semin. Immunopathol., 34: 787-792.
- Khan, M.B., J.W.K. Liew, C.S. Leong and Y.L. Lau, 2016. Role of NF-kβ factor Rel2 during Plasmodium falciparum and bacterial infection in Anopheles dirus. Parasites Vectors, 9.
- Ramirez, J.L., L.S. Garver, F.A. Brayner, L.C. Alves, J. Rodrigues, A. Molina-Cruz and C. Barillas-Mury, 2014. The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate Immun., 6: 119-128.
- Smith, R.C., J.G. King, D. Tao, O.A. Zeleznik, C. Brando, G.G. Thallinger and R.R. Dinglasan, 2016. Molecular profiling of phagocytic immune cells in Anopheles gambiae reveals integral roles for hemocytes in mosquito innate immunity. Mol. Cell. Proteomics, 15: 3373-3387.
- Eleftherianos, I., C. Heryanto, T. Bassal, W. Zhang, G. Tettamanti and A. Mohamed, 2021. Haemocyte‐mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology, 164: 401-432.
- Molina-Cruz, A., R.J. DeJong, B. Charles, L. Gupta, S. Kumar, G. Jaramillo-Gutierrez and C. Barillas-Mury, 2008. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and plasmodium. J. Biol. Chem., 283: 3217-3223.
- Chandley, P., R. Ranjan, S. Kumar and S. Rohatgi, 2022. Host-parasite interactions during Plasmodium infection: Implications for immunotherapies. Front. Immunol., 13.
- Silva-Gomes, S., A. Decout and J. Nigou, 2014. Pathogen-Associated Molecular Patterns (PAMPs). In: Compendium of Inflammatory Diseases, Parnham, M.J. (Ed.), Springer, Basel, ISBN: 978-3-0348-0620-6, pp: 1-16.
- Smith, R.C., C. Barillas-Mury and M. Jacobs-Lorena, 2015. Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A., 112: E3412-E3420.
- Garver, L.S., Y. Dong and G. Dimopoulos, 2009. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog., 5.
- Kurup, S.P., N.S. Butler and J.T. Harty, 2019. T cell-mediated immunity to malaria. Nat. Rev. Immunol., 19: 457-471.
- Rénia, L. and Y.S. Goh, 2016. Malaria parasites: The great escape. Front. Immunol., 7.
- Guilbride, D.L., P.D.L. Guilbride and P. Gawlinski, 2012. Malaria's deadly secret: A skin stage. Trends Parasitol., 28: 142-150.
- Gomes, P.S., J. Bhardwaj, J. Rivera-Correa, C.G. Freire-De-Lima and A. Morrot, 2016. Immune escape strategies of malaria parasites. Front. Microbiol., 7.
- Wang, C., X. Liu, Z. Li, Y. Chai and Y. Jiang et al., 2015. CD8+NKT-like cells regulate the immune response by killing antigen-bearing DCs. Sci. Rep., 5.
- Zheng, H., Z. Tan and W. Xu, 2014. Immune evasion strategies of pre-erythrocytic malaria parasites. Mediators Inflammation, 2014.
- Dinko, B. and G. Pradel, 2016. Immune evasion by Plasmodium falciparum parasites: Converting a host protection mechanism for the parasite′s benefit. Adv. Infect. Dis., 6: 82-95.
- He, X., L. Xia, K.C. Tumas, J. Wu and X.Z. Su, 2020. Type I interferons and malaria: A double-edge sword against a complex parasitic disease. Front. Cell. Infect. Microbiol., 10.
- Risco-Castillo, V., S. Topçu, C. Marinach, G. Manzoni and A.E. Bigorgne et al., 2015. Malaria sporozoites traverse host cells within transient vacuoles. Cell Host Microbe, 18: 593-603.
- Holz, L.E., D. Fernandez-Ruiz and W.R. Heath, 2016. Protective immunity to liver-stage malaria. Clin. Transl. Immunol., 5.
- Casanova, J.L., L. Abel and L. Quintana-Murci, 2011. Human TLRs and IL-1Rs in host defense: Natural insights from evolutionary, epidemiological, and clinical genetics. Annu. Rev. Immunol., 29: 447-491.
- Dennison, N.J., O.J. BenMarzouk-Hidalgo and G. Dimopoulos, 2015. MicroRNA-regulation of Anopheles gambiae immunity to Plasmodium falciparum infection and midgut microbiota. Dev. Comp. Immunol., 49: 170-178.
- Peymanfar, Y. and A.W. Taylor-Robinson, 2016. Plasmodium sexual stage parasites present distinct targets for malaria transmission-blocking vaccine design. Int. J. Vaccine Immunization, 2.
- Wang, Y.Y., W. Hu, F.S. Wang and C. Zhang, 2022. Revisiting the role of human memory CD8+ T cells in immune surveillance. Cell. Mol. Immunol., 19: 1319-1321.
- Dups, J.N., M. Pepper and I.A. Cockburn, 2014. Antibody and B cell responses to Plasmodium sporozoites. Front. Microbiol., 5.
- Bertolino, P. and D.G. Bowen, 2015. Malaria and the liver: Immunological hide-and-seek or subversion of immunity from within? Front. Microbiol., 6.
- Fortin, A., M.M. Stevenson and P. Gros, 2002. Susceptibility to malaria as a complex trait: Big pressure from a tiny creature. Hum. Mol. Genet., 11: 2469-2478.
- Arama, C., P. Giusti, S. Boström, V. Dara and B. Traore et al., 2011. Interethnic differences in antigen-presenting cell activation and TLR responses in malian children during Plasmodium falciparum malaria. PLoS ONE, 6.
- Portugal, S., C. Carret, M. Recker, A.E. Armitage and L.A. Gonçalves et al., 2011. Host-mediated regulation of superinfection in malaria. Nat. Med., 17: 732-737.
- Kariuki, S.N. and T.N. Williams, 2020. Human genetics and malaria resistance. Hum. Genet., 139: 801-811.
- Kariuki, S.N., A. Marin-Menendez, V. Introini, B.J. Ravenhill and Y.C. Lin et al., 2020. Red blood cell tension protects against severe malaria in the Dantu blood group. Nature, 585: 579-583.
- Band, G., Q.S. Le, G.M. Clarke, K. Kivinen and C. Hubbart et al., 2019. Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania. Nat. Commun., 10.
- Williams, T.N., 2016. Host Genetics. In: Advances in Malaria Research, Gaur, D., C.E. Chitnis and V.S. Chauhan (Eds.), John Wiley & Sons, Inc., Hoboken, New Jersey, ISBN: 9781118493816, pp: 465-494.
- Band, G., K.A. Rockett, C.C.A. Spencer, D.P. Kwiatkowski and B. Gavin et al., 2015. A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature, 526: 253-257.
- Leffler, E.M., G. Band, G.B.J. Busby, K. Kivinen and Q.S. Le et al., 2017. Resistance to malaria through structural variation of red blood cell invasion receptors. Science, 356.
- Ndila, C.M., S. Uyoga, A.W. Macharia, G. Nyutu and N. Peshu et al., 2018. Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: A case-control association study. Lancet Haematol., 5: e333-e345.
- Anstee, D.J., 1990. Blood group-active surface molecules of the human red blood cell. Vox Sang., 58: 1-20.
- Zerihun, T., A. Degarege and B. Erko, 2011. Association of ABO blood group and Plasmodium falciparum malaria in Dore Bafeno Area, Southern Ethiopia. Asian Pac. J. Trop. Biomed., 1: 289-294.
- Acquah, F.K., D. Donu, D. Bredu, S. Eyia-Ampah and J.A. Amponsah et al., 2020. Asymptomatic carriage of Plasmodium falciparum by individuals with variant blood groups and haemoglobin genotypes in Southern Ghana. Malar. J., 19.
- Kwabi-Addo, B., 2017. Health Outcomes in a Foreign Land. 1st Edn., Springer International Publishing, Cham, Switzerland, ISBN: 978-3-319-55864-6, Pages: 324.
- Degarege, A., M.T. Gebrezgi, G. Ibanez, M. Wahlgren and P. Madhivanan, 2019. Effect of the ABO blood group on susceptibility to severe malaria: A systematic review and meta-analysis. Blood Rev., 33: 53-62.
- Makkawi, M., S. Alasmari, A.A. Hawan, M.M. Al Shahrani and A.A. Dera, 2021. Hemoglobinopathies: An update on the prevalence trends in Southern Saudi Arabia. Saudi Med. J., 42: 784-789.
- Karakochuk, C.D., S.Y. Hess, D. Moorthy, S. Namaste and M.E. Parker et al., 2019. Measurement and interpretation of hemoglobin concentration in clinical and field settings: A narrative review. Ann. New York Acad. Sci., 1450: 126-146.
- Okoh, M.P., L.A. Alli, M.E.E. Tolvanen and M.M. Nwegbu, 2019. Herbal drug use in sickle cell disease management; Trends and perspectives in sub-saharan africa-A systematic review. Curr. Drug Disc. Technol., 16: 372-385.
- Hannemann, A., E. Weiss, D.C. Rees, S. Dalibalta, J.C. Ellory and J.S. Gibson, 2011. The properties of red blood cells from patients heterozygous for HbS and HbC (HbSC genotype). Anemia, 2011.
- Williams, T.N., T.W. Mwangi, D.J. Roberts, N.D. Alexander and D.J. Weatherall et al., 2005. An immune basis for malaria protection by the sickle cell trait. PLoS. Med., 2.
- Duffy, P.E. and M. Fried, 2006. Red blood cells that do and red blood cells that don't: How to resist a persistent parasite. Trends Parasitol., 22: 99-101.
- Petersen, J.E.V., J.W. Saelens, E. Freedman, L. Turner and T. Lavstsen et al., 2021. Sickle-trait hemoglobin reduces adhesion to both CD36 and EPCR by Plasmodium falciparum-infected erythrocytes. PLoS Pathog., 17.
- Ayi, K., F. Turrini, A. Piga and P. Arese, 2004. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: A common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood, 104: 3364-3371.
- Cholera, R., N.J. Brittain, M.R. Gillrie, T.M. Lopera-Mesa and S.A.S. Diakité et al., 2008. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. U.S.A., 105: 991-996.
- Vichinsky, E.P., 2009. Alpha thalassemia major-new mutations, intrauterine management and outcomes. Hematol. Am. Soc. Hematol. Educ. Progr., 2009: 35-41.
- Viprakasit, V. and S. Ekwattanakit, 2018. Clinical classification, screening and diagnosis for thalassemia. Hematol. Oncol. Clin. North Am., 32: 193-211.
- Wambua, S., T.W. Mwangi, M. Kortok, S.M. Uyoga and A.W. Macharia et al., 2006. The effect of α+-thalassaemia on the incidence of malaria and other diseases in children living on the Coast of Kenya. PLoS Med., 3.
- Krause, M.A., S.A.S. Diakite, T.M. Lopera-Mesa, C. Amaratunga and T. Arie et al., 2012. α-Thalassemia impairs the cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS ONE, 7.
- Jamerson, B.D., T.H. Haryadi and A. Bohannon, 2020. Glucose-6-phosphate dehydrogenase deficiency: An actionable risk factor for patients with COVID-19? Arch. Med. Res., 51: 743-744.
- Tripathy, V. and B.M. Reddy, 2007. Present status of understanding on the G6PD deficiency and natural selection. J. Postgrad. Med., 53: 193-202.
- Tougan, T., J.R. Edula, M. Morita, E. Takashima, H. Honma, T. Tsuboi and T. Horii, 2020. The malaria parasite Plasmodium falciparum in red blood cells selectively takes up serum proteins that affect host pathogenicity. Malar. J., 19.
- Louicharoen, C., E. Patin, R. Paul, I. Nuchprayoon and B. Witoonpanich et al., 2009. Positively selected G6PD-mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science, 326: 1546-1549.
- Grant, A.V., C. Roussilhon, R. Paul and A. Sakuntabhai, 2015. The genetic control of immunity to Plasmodium infection. BMC Immunol., 16.
- Nakamura, T., T. Shirouzu, K. Nakata, N. Yoshimura and H. Ushigome, 2019. The role of major histocompatibility complex in organ transplantation-donor specific anti-major histocompatibility complex antibodies analysis goes to the next stage. Int. J. Mol. Sci., 20.
- Crux, N.B. and S. Elahi, 2017. Human leukocyte antigen (HLA) and immune regulation: How do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections? Front. Immunol., 8.
- Sanchez-Mazas, A., V. Černý, D. Di, S. Buhler and E. Podgorná et al., 2017. The HLA-B landscape of Africa: Signatures of pathogen-driven selection and molecular identification of candidate alleles to malaria protection. Mol. Ecol., 26: 6238-6252.
- Osafo-Addo, A.D., K.A. Koram, A.R. Oduro, M. Wilson, A. Hodgson and W.O. Rogers, 2008. HLA-DRB1*04 allele is associated with severe malaria in Northern Ghana. Am. J. Trop. Med. Hyg., 78: 251-255.
- Mackinnon, M.J., T.W. Mwangi, R.W. Snow, K. Marsh and T.N. Williams, 2005. Heritability of malaria in Africa. PLoS Med., 2.
- Sultana, M., N. Sheikh, R.A. Mahumud, T. Jahir, Z. Islam and A.R. Sarker, 2017. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop. Med. Health, 45.
- Portugal, S., D. Doumtabe, B. Traore, L.H. Miller and M. Troye-Blomberg et al., 2012. B cell analysis of ethnic groups in Mali with differential susceptibility to malaria. Malar. J., 11.
- Arama, C., B. Maiga, A. Dolo, B. Kouriba and B. Traoré et al., 2015. Ethnic differences in susceptibility to malaria: What have we learned from immuno-epidemiological studies in West Africa? Acta Trop., 146: 152-156.
- Torcia, M.G., V. Santarlasci, L. Cosmi, A. Clemente and L. Maggi et al., 2008. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. U.S.A., 105: 646-651.
- Farouk, S.E., A. Dolo, S. Bereczky, B. Kouriba and B. Maiga et al., 2005. Different antibody- and cytokine-mediated responses to Plasmodium falciparum parasite in two sympatric ethnic tribes living in Mali. Microbes Infect., 7: 110-117.
- Perez, L.G., M.R. Costa, C.A. Todd, B.F. Haynes and D.C. Montefiori, 2009. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane-proximal external region of gp41. J. Virol., 83: 7397-7410.
- Israelsson, E., M. Vafa, B. Maiga, A. Lysén and N.C. Iriemenam et al., 2008. Differences in Fcgamma receptor IIa genotypes and IgG subclass pattern of anti-malarial antibodies between sympatric ethnic groups in Mali. Malar. J., 7.
- Willcocks, L.C., E.J. Carr, H.A. Niederer, T.F. Rayner and T.N. Williams et al., 2010. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. U.S.A., 107: 7881-7885.
- Adu-Gyasi, D., M. Adams, S. Amoako, E. Mahama and M. Nsoh et al., 2012. Estimating malaria parasite density: Assumed white blood cell count of 10,000/μl of blood is appropriate measure in Central Ghana. Malar. J., 11.
- Zhao, J., L. Ma, S. Chen, Y. Xie and L. Xie et al., 2014. Association between Fc-gamma receptor IIa (CD32) gene polymorphism and malaria susceptibility: A meta-analysis based on 6928 subjects. Infect. Genet. Evol., 23: 169-175.
- Furini, A.A.C., M.P. Capobianco, L.M. Storti-Melo, M.G. Cunha, G.C. Cassiano and R.L.D. Machado, 2016. Cytokine gene polymorphisms are not associated with anti-PvDBP, anti-PvAMA-1 or anti-PvMSP-119 IgG antibody levels in a malaria-endemic area of the Brazilian Amazon. Malar. J., 15.
- Lokossou, A.G., C. Dechavanne, A. Bouraïma, D. Courtin and A.L. Port et al., 2013. Association of IL-4 and IL-10 maternal haplotypes with immune responses to P. falciparum in mothers and newborns. BMC Infect. Dis., 13.
- Fraley, E., C. LeMaster, S. Khanal, D. Banerjee and T. Pastinen et al., 2021. The impact of prior infection and age on antibody persistence after severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine. Clin. Infect. Dis., 75: e902-e904.
- Aka, K.G., S.S. Yao, E.A. Gbessi, A.M. Adja and V. Corbel et al., 2021. Influence of host-related factors and exposure to mosquito bites on the dynamics of antibody response to Plasmodium falciparum antigens. TropicalMed, 6.
- Osier, F.H.A., G. Fegan, S.D. Polley, L. Murungi and F. Verra et al., 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun., 76: 2240-2248.
- Rono, J., F.H.A. Osier, D. Olsson, S. Montgomery and L. Mhoja et al., 2013. Breadth of anti-merozoite antibody responses is associated with the genetic diversity of asymptomatic Plasmodium falciparum infections and protection against clinical malaria. Clin. Infect. Dis., 57: 1409-1416.
- Garcia-Senosiain, A., I.H. Kana, S.K. Singh, B.K. Chourasia and M.K. Das et al., 2020. Peripheral merozoite surface proteins are targets of naturally acquired immunity against malaria in both India and Ghana. Infect. Immun., 88.
- Reiling, L., M.J. Boyle, M.T. White, D.W. Wilson and G. Feng et al., 2019. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat. Commun., 10.
- Yman, V., M.T. White, M. Asghar, C. Sundling and K. Sondén et al., 2019. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med., 17.
- Mayengue, P.I., D.K. Batsimba, R.F. Niama, R.I. Ottia and A. Malonga-Massanga et al., 2020. Variation of prevalence of malaria, parasite density and the multiplicity of Plasmodium falciparum infection throughout the year at three different health centers in Brazzaville, Republic of Congo. BMC Infect. Dis., 20.
- Fong, S.W., R.M. Kini and L.F.P. Ng, 2018. Mosquito saliva reshapes alphavirus infection and immunopathogenesis. J. Virol., 92.
- MacGillivray, D.M. and T.R. Kollmann, 2014. The role of environmental factors in modulating immune responses in early life. Front. Immunol., 5.
- van der Heijden, C.D.C.C., M.P. Noz, L.A.B. Joosten, M.G. Netea, N.P. Riksen and S.T. Keating, 2018. Epigenetics and trained immunity. Antioxid. Redox Signaling, 29: 1023-1040.
- Tripet, F., F. Aboagye-Antwi and H. Hurd, 2008. Ecological immunology of mosquito-malaria interactions. Trends Parasitol., 24: 219-227.
- Chan, J.A., K.B. Howell, L. Reiling, R. Ataide and C.L. Mackintosh et al., 2012. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest., 122: 3227-3238.
- Tepa, A., I. Abame, V. Makamta, B. Fongang, J. Donkeu, L. Ayong and C.A. Pieme, 2020. Nutritional status and humoral immune response to Plasmodium falciparum in children aged 6-59 months. J. Trop. Med., 2020.
- Henchion, M., M. Hayes, A.M. Mullen, M. Fenelon and B. Tiwari, 2017. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods, 6.
- Nunes-Cabaço, H., D. Moita, C. Rôla, A.M. Mendes and M. Prudêncio, 2022. Impact of dietary protein restriction on the immunogenicity and efficacy of whole-sporozoite malaria vaccination. Front. Immunol., 13.
- Tan, J., D. Ni, J. Taitz, G.V. Pinget and M. Read et al., 2022. Dietary protein increases T-cell-independent sIgA production through changes in gut microbiota-derived extracellular vesicles. Nat. Commun., 13.
- Pezo, R.C., M. Wong and A. Martin, 2019. Impact of the gut microbiota on immune checkpoint inhibitor-associated toxicities. Ther. Adv. Gastroenterol., 12.
- Kaye, D. and A. Isidori, 2021. Current challenges in hematology: Awareness, prevention, equity. Front. Oncol., 11.
- Graça, D., V. Brglez, J. Allouche, K. Zorzi and C. Fernandez et al., 2023. Both humoral and cellular immune responses to SARS-CoV-2 are essential to prevent infection: A prospective study in a working vaccinated population from Southern France. J. Clin. Immunol., 43.
- Murdock, C.C., K.P. Paaijmans, A.S. Bell, J.G. King, J.F. Hillyer, A.F. Read and M.B. Thomas, 2012. Complex effects of temperature on mosquito immune function. Proc. Biol. Sci., 279: 3357-3366.
- Furci, L. and M. Secchi, 2018. AMPs and Mechanisms of Antimicrobial Action. In: Antimicrobial Peptides in Gastrointestinal Diseases, Cho, C.H. (Ed.), Academic Press, Cambridge, Massachusetts, ISBN: 9780128143193, pp: 97-131.
- Ferreira, P.G., B. Tesla, E.C.A. Horácio, L.A. Nahum, M.A. Brindley, T.A. de Oliveira Mendes and C.C. Murdock, 2020. Temperature dramatically shapes mosquito gene expression with consequences for mosquito-zika virus interactions. Front. Microbiol., 11.
- Murdock, C.C., E.D. Sternberg and M.B. Thomas, 2016. Malaria transmission potential could be reduced with current and future climate change. Sci. Rep., 6.
- Le, A., A.A. King, F.M.G. Magpantay, A. Mesbahi and P. Rohani, 2021. The impact of infection-derived immunity on disease dynamics. J. Math. Biol., 83.
- van Seventer, J.M. and N.S. Hochberg, 2017. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. In: International Encyclopedia of Public Health, Quah, S.R. (Ed.), Academic Press, Cambridge, Massachusetts, ISBN: 9780128037089, pp: 22-39.
- Burrell, C.J., C.R. Howard and F.A. Murphy, 2017. Epidemiology of Viral Infections. In: Fenner and White's Medical Virology, Burrell, C.J., C.R. Howard and F.A. Murphy (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780123751560, pp: 185-203.
- van den Driessche, P., 2017. Reproduction numbers of infectious disease models. Infect. Dis. Modell., 2: 288-303.
- Salazar-Castañon, V.H., M. Legorreta-Herrera and M. Rodriguez-Sosa, 2014. Helminth parasites alter protection against Plasmodium infection. BioMed Res. Int., 2014.
- Maizels, R.M. and H.J. McSorley, 2016. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol., 138: 666-675.
- Salgame, P., G.S. Yap and W.C. Gause, 2013. Effect of helminth-induced immunity on infections with microbial pathogens. Nat. Immunol., 14: 1118-1126.
- Nutman, T.B., 2015. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol., 37: 304-313.
- Alli, L.A., A.A. Adesokan and A.O. Salawu, 2016. Antimalarial activity of fractions of aqueous extract of Acacia nilotica root. J. Intercultural Ethnopharmacol., 5: 180-185.
- Su, X.Z., C. Zhang and D.A. Joy, 2020. Host-malaria parasite interactions and impacts on mutual evolution. Front. Cell. Infect. Microbiol., 10.
- Edwards, H.M., H. Counihan, C. Bonnington, J. Achan, P. Hamade and J.K. Tibenderana, 2021. The impact of malaria coinfection on Ebola virus disease outcomes: A systematic review and meta-analysis. PLoS ONE, 16.
- Teo, T.H., F.M. Lum, K. Ghaffar, Y.H. Chan and S.N. Amrun et al., 2018. Plasmodium co-infection protects against chikungunya virus-induced pathologies. Nat. Commun., 9.
- Gonçalves, R.M., N.F. Lima and M.U. Ferreira, 2014. Parasite virulence, co-infections and cytokine balance in malaria. Pathog. Global Health, 108: 173-178.
- Craig, A.G., G.E. Grau, C. Janse, J.W. Kazura and dan Milner et al., 2012. The role of animal models for research on severe malaria. PLoS Pathog., 8.
- Church, J. and K. Maitland, 2014. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: A systematic review. BMC Med., 12.
- Aitken, E.H., A. Alemu and S.J. Rogerson, 2018. Neutrophils and malaria. Front. Immunol., 9.
- Snow, R.W., P. Amratia, C.W. Kabaria, A.M. Noor and K. Marsh, 2012. The Changing Limits and Incidence of Malaria in Africa: 1939-2009. In: Advances in Parasitology, Rollinson, D. and S.I. Hay (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780123943033, pp: 169-162.
- Alemu, M., M. Ayana, H. Abiy, B. Minuye, W. Alebachew and A. Endalamaw, 2019. Determinants of neonatal sepsis among neonates in the northwest part of Ethiopia: Case-control study. Ital. J. Pediatr., 45. https://doi.org/10.1186/s13052-019-0739-2
- Nikolopoulos, G.K. and A.G. Tsantes, 2022. Recent HIV infection: Diagnosis and public health implications. Diagnostics, 12.
- Del-Tejo, P.L., N. Cubas-Vega, C. Caraballo-Guerra, B.M. da Silva and J. da Silva Valente et al., 2021. Should we care about Plasmodium vivax and HIV co-infection? A systematic review and a cases series from the Brazilian Amazon. Malar. J., 20.
- Nyabadza, F., B.T. Bekele, M.A. Rúa, D.M. Malonza, N. Chiduku and M. Kgosimore, 2015. The implications of HIV treatment on the HIV-malaria coinfection dynamics: A modeling perspective. BioMed Res. Int., 2015.
- van Geertruyden, J.P., M. Mulenga, L. Mwananyanda, V. Chalwe and F. Moerman et al., 2006. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J. Infect. Dis., 194: 917-925.
- Kwenti, T.E., 2018. Malaria and HIV coinfection in sub-Saharan Africa: Prevalence, impact, and treatment strategies. Res. Rep. Trop. Med., 9: 123-136.
- Munyenyembe, A.U., K. Gausi, T.S. Nyirenda, J. Hiestand, J. Mallewa and W.L. Mandala, 2018. HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria. J. Blood Med., 9: 153-162.
- Chavale, H., J.R. Santos-Oliveira, A.M. da-Cruz and S. Enosse, 2012. Enhanced T cell activation in Plasmodium falciparum malaria-infected human immunodeficiency virus-1 patients from Mozambique. Mem. Inst. Oswaldo Cruz, 107: 985-992.
- Byakika-Kibwika, P., M. Lamorde, J. Mayito, L. Nabukeera and R. Namakula et al., 2012. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J. Antimicrob. Chemother., 67: 2213-2221.
- Loke, P., S.C. Lee and O.O. Oyesola, 2022. Effects of helminths on the human immune response and the microbiome. Mucosal Immunol., 15: 1224-1233.
- Jegede, F.E., T.I. Oyeyi, S.A. Abdulrahman, H.A. Mbah, T. Badru, C. Agbakwuru and O. Adedokun, 2017. Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano, Nigeria. PLoS ONE, 12.
- McSorley, H.J. and R.M. Maizels, 2012. Helminth infections and host immune regulation. Clin. Microbiol. Rev., 25: 585-608.
- Aliota, M.T., C.C. Chen, H. Dagoro, J.F. Fuchs and B.M. Christensen, 2011. Filarial worms reduce Plasmodium infectivity in mosquitoes. PLoS Negl. Trop. Dis., 5.
- Mabbott, N.A., 2018. The influence of parasite infections on host immunity to co-infection with other pathogens. Front. Immunol., 9.
- Inclan-Rico, J.M. and M.C. Siracusa, 2018. First responders: Innate immunity to helminths. Trends Parasitol., 34: 861-880.
- Gerbe, F., E. Sidot, D.J. Smyth, M. Ohmoto and I. Matsumoto et al., 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature, 529: 226-230.
- Gurung, P. and T.D. Kanneganti, 2016. Immune responses against protozoan parasites: A focus on the emerging role of nod-like receptors. Cell. Mol. Life Sci., 73: 3035-3051.
- Perez-Mazliah, D. and J. Langhorne, 2014. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front. Immunol., 5.
- Bruno, M.E.C., A.L. Frantz, E.W. Rogier, F.E. Johansen and C.S. Kaetzel, 2011. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-κB pathways in intestinal epithelial cells. Mucosal Immunol., 4: 468-478.
- Guerrero-Bosagna, C., 2017. Epigenetics, evolution and the survival of the non-unfit. Biochemist, 39: 8-11.
- Yasui, F., C. Kai, K. Saito, S. Inoue and M. Yoneda et al., 2010. Analysis of the mechanism by which BALB/c mice having prior immunization with nucleocapsid protein of SARS-CoV develop severe pneumonia after SARS-CoV infection. Procedia Vaccinol., 2: 44-50.
- Cirimotich, C.M., Y. Dong, L.S. Garver, S. Sim and G. Dimopoulos, 2010. Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol., 34: 387-395.
- Hoeve, M.A., K.J. Mylonas, K.J. Fairlie-Clarke, S.M. Mahajan, J.E. Allen and A.L. Graham, 2009. Plasmodium chabaudi limits early Nippostrongylus brasiliensis-induced pulmonary immune activation and Th2 polarization in co-infected mice. BMC Immunol., 10.
- Karadjian, G., D. Berrebi, N. Dogna, N. Vallarino-Lhermitte, O. Bain, I. Landau and C. Martin, 2014. Co-infection restrains Litomosoides sigmodontis filarial load and plasmodial P. yoelii but not P. chabaudi parasitaemia in mice. Parasite, 21.
- Colley, D.G., A.L. Bustinduy, W.E. Secor and C.H. King, 2014. Human schistosomiasis. Lancet, 383: 2253-2264.
- Pearce, E.J. and A.S. MacDonald, 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol., 2: 499-511.
- Colley, D.G. and W.E. Secor, 2014. Immunology of human schistosomiasis. Parasite Immunol., 36: 347-357.
- Karthik, L., G. Kumar, T. Keswani, A. Bhattacharyya, S.S. Chandar and K.B. Rao, 2014. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PloS One, 9.
- Abdulkadir, A., I. Umar, S. Ibrahim, E. Onyike and A. Kabiru, 2016. Cysteine protease inhibitors from Calotropis procera with antiplasmodial potential in mice. J. Adv. Med. Pharm. Sci., 6.
- Bickle, Q.D., J. Solum and H. Helmby, 2008. Chronic intestinal nematode infection exacerbates experimental Schistosoma mansoni infection. Infect. Immun., 76: 5802-5809.
- Beechler, B.R., A.E. Jolles, S.A. Budischak, P.L.A.M. Corstjens and V.O. Ezenwa et al., 2017. Host immunity, nutrition and coinfection alter longitudinal infection patterns of schistosomes in a free ranging African buffalo population. PLoS Negl. Trop. Dis., 11.
- Bazzone, L.E., P.M. Smith, L.I. Rutitzky, M.G. Shainheit and J.F. Urban et al., 2008. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect. Immun., 76: 5164-5172.
- Rudenko, G., 2011. African trypanosomes: The genome and adaptations for immune evasion. Essays Biochem., 51: 47-62.
- Tabel, H., G. Wei and H.J. Bull, 2013. Immunosuppression: Cause for failures of vaccines against African trypanosomiases. PLoS Negl. Trop. Dis., 7.
- Caljon, G., N. van Reet, C. de Trez, M. Vermeersch, D. Pérez-Morga and J. van den Abbeele, 2016. The dermis as a delivery site of Trypanosoma brucei for tsetse flies. PLoS Pathog., 12.
- Ademola, I.O. and P.O. Odeniran, 2016. Co-infection with Plasmodium berghei and Trypanosoma brucei increases severity of malaria and trypanosomiasis in mice. Acta Trop., 159: 29-35.
- Machelart, A., M. van Vyve, G. Potemberg, A. Demars and C. de Trez et al., 2017. Trypanosoma infection favors Brucella elimination via IL-12/IFNγ-dependent pathways. Front. Immunol., 8.
- Bretscher, P.A., 2014. The activation and inactivation of mature
CD 4T cells: A case for peripheral self-nonself discrimination. Scand. J. Immunol., 79: 348-360. - Akoolo, L., S.C. Rocha and N. Parveen, 2022. Protozoan co-infections and parasite influence on the efficacy of vaccines against bacterial and viral pathogens. Front. Microbiol., 13.
- Reynolds, L.A., S.A. Redpath, S. Yurist-Doutsch, N. Gill and E.M. Brown et al., 2017. Enteric helminths promote Salmonella coinfection by altering the intestinal metabolome. J. Infect. Dis., 215: 1245-1254.
- Khan, I.A., R. Hakak, K. Eberle, P. Sayles, L.M. Weiss and J.F. Urban Jr., 2008. Coinfection with Heligmosomoides polygyrus fails to establish CD8+ T-Cell Immunity against Toxoplasma gondii. Infect. Immun., 76: 2256-2256.
- Silva, R.C.M.C., L.H. Travassos, C.N. Paiva and M.T. Bozza, 2020. Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance. PLoS Pathog., 16.
- Mtove, G., B. Amos, L. von Seidlein, I. Hendriksen and A. Mwambuli et al., 2010. Invasive salmonellosis among children admitted to a rural Tanzanian hospital and a comparison with previous studies. PLoS ONE, 5.
- Gozzelino, R., V. Jeney and M.P. Soares, 2010. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol., 50: 323-354.
- Cunnington, A.J., M. Njie, S. Correa, E.N. Takem, E.M. Riley and M. Walther, 2012. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J. Immunol., 189: 5336-5346.
- Osborne, L.C., L.A. Monticelli, T.J. Nice, T.E. Sutherland and M.C. Siracusa et al., 2014. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science, 345: 578-582.
- Reese, P.P., R.D. Bloom, H.I. Feldman, P. Rosenbaum and W. Wang et al., 2014. Mortality and cardiovascular disease among older live kidney donors. Am. J. Transplant., 14: 1853-1861.
- Rubio, M., Q. Bassat, X. Estivill and A. Mayor, 2016. Tying malaria and microRNAs: From the biology to future diagnostic perspectives. Malar. J., 15.
- Morawski, B.M., M. Yunus, E. Kerukadho, G. Turyasingura and L. Barbra et al., 2017. Hookworm infection is associated with decreased CD4+ T cell counts in HIV-infected adult Ugandans. PLoS Negl. Trop. Dis., 11.
- Connick, K., R. Lalor, A. Murphy, A. Glasgow and C. Breen et al., 2022. RNA-seq analysis of murine peyer’s patches at 6 and 18 h post infection with Fasciola hepatica metacecariae. Vet. Parasitol., 302.
- Dietze, K.K., U. Dittmer, D.K. Koudaimi, S. Schimmer, M. Reitz, M. Breloer and W. Hartmann, 2016. Filariae-retrovirus co-infection in mice is associated with suppressed virus-specific IgG immune response and higher viral loads. PLoS Negl. Trop. Dis., 10.
- McGovern, K. and E. Wilson, 2013. Role of chemokines and trafficking of immune cells in parasitic infections. Curr. Immunol. Rev., 9: 157-168.
- Vijayan, K., L. Wei, E.K.K. Glennon, C. Mattocks, N. Bourgeois, B. Staker and A. Kaushansky, 2021. Host-targeted interventions as an exciting opportunity to combat malaria. Chem. Rev., 121: 10452-10468.
- Dzomba, P., T. Chayamiti, S. Nyoni, P. Munosiyei and I. Gwizangwe, 2012. Ferriprotoporphyrin IX-Combretum imberbe crude extracts interactions: Implication for malaria treatment. Afr. J. Pharm. Pharmacol., 6: 2205-2210.
- Raphemot, R., M. Toro-Moreno, K.Y. Lu, D. Posfai and E.R. Derbyshire, 2019. Discovery of druggable host factors critical to Plasmodium liver-stage infection. Cell Chem. Biol., 26: 1253-1262.
- Loiseau, C., M.M. Cooper and D.L. Doolan, 2020. Deciphering host immunity to malaria using systems immunology. Immunol. Rev., 293: 115-143.
- Gulbis, B., F. Cotton and F. Vertongen, 2004. Rare abnormal hemoglobins. EMC-Hématologie, 1: 106-114.
- Moore, L.R., H. Fujioka, P.S. Williams, J.J. Chalmers, B. Grimberg, P.A. Zimmerman and M. Zborowski, 2006. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. FASEB J., 20: 747-749.
- Kato, N., E. Comer, T. Sakata-Kato, A. Sharma and M. Sharma et al., 2016. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature, 538: 344-349.
How to Cite this paper?
APA-7 Style
Adejoh,
J., Madu,
C., Egua,
M.O., Alli,
L.A., Raheem,
D., Okoh,
M.P. (2023). Molecular and Epigenetics Mechanisms for the Immune Control of Plasmodium Parasites Infection: A Comprehensive Review. Asian Journal of Biological Sciences, 16(4), 636-669. https://doi.org/10.3923/ajbs.2023.636.669
ACS Style
Adejoh,
J.; Madu,
C.; Egua,
M.O.; Alli,
L.A.; Raheem,
D.; Okoh,
M.P. Molecular and Epigenetics Mechanisms for the Immune Control of Plasmodium Parasites Infection: A Comprehensive Review. Asian J. Biol. Sci 2023, 16, 636-669. https://doi.org/10.3923/ajbs.2023.636.669
AMA Style
Adejoh
J, Madu
C, Egua
MO, Alli
LA, Raheem
D, Okoh
MP. Molecular and Epigenetics Mechanisms for the Immune Control of Plasmodium Parasites Infection: A Comprehensive Review. Asian Journal of Biological Sciences. 2023; 16(4): 636-669. https://doi.org/10.3923/ajbs.2023.636.669
Chicago/Turabian Style
Adejoh, Johnson, Chijioke Madu, Maxwell Osaronowen Egua, Lukman Adewale Alli, Dele Raheem, and Michael Prince Okoh.
2023. "Molecular and Epigenetics Mechanisms for the Immune Control of Plasmodium Parasites Infection: A Comprehensive Review" Asian Journal of Biological Sciences 16, no. 4: 636-669. https://doi.org/10.3923/ajbs.2023.636.669

This work is licensed under a Creative Commons Attribution 4.0 International License.



