Chemical and Some Biochemical Assessments of Various Parts of Passion Fruit (Passiflora edulis Sims) Plant
| Received 20 Jan, 2022 |
Accepted 23 Dec, 2023 |
Published 31 Mar, 2024 |
Background and Objective: The medicinal importance of plants and plant products have been of global relevance and interest. However, this study sought to investigate the proximate and antinutrients compositions, nutritional viable minerals evaluation and in vitro antioxidants assessments of the Passiflora edulis plant. Materials and Methods: Fresh samples of the plant of passion fruit (Passiflora edulis Sims) were obtained and separated into the leaf, leaf stalk, fruit pod and fruit seed which were air-dried, crushed and subsequently ground to powder and assessed for various parameters using standard methods. Results: The proximate analysis showed that nutritional compounds were well distributed in different concentrations in the plant. The antinutrients factors of phytate, oxalate and tannin were present in varying concentrations in the plant. The results also showed the leaves and seeds contained higher concentrations of vitamin C than other parts of the plant. The in vitro antioxidant analyses of various parts of the plant showed that all the parts possessed the ability to scavenge DPPH and ABTS free radicals, ferric reducing antioxidant power with iron chelation potential as well as good concentrations of flavonoids and total phenolic contents in varying percentages and concentrations in all the parts of the plant. It was also evidenced in the results that all the parts of the plant contained macro and micro nutritional viable elements like iron, sodium, calcium, zinc, manganese, nickel, cobalt and copper as well as lead except chromium and cadmium that were not detectable. Conclusion: It could also be seen from the results that all the parts of the plant are potentially good sources of nutritional and medicinally important compounds and can be utilized in animal feeds composition.
| Copyright © 2024 Abass et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Passion fruit just like many other fruits is an important source of many nutrients, including calcium, fibre, vitamin C and folic acid. These nutrients in fruits are vital for the health and maintenance of the human body. The Passiflora genus was said to comprise about 500 species, which was believed to be the largest in the family Passifloraceae. The Passiflora edulis stands out and is unique in the family of Passifloraceae as a result of its economic and medicinal usefulness1. The plant of Passiflora edulis is widely propagated in tropical and subtropical regions in numerous parts of the world, especially in South America, the Caribbean, South Florida, South Africa and Asia2-4. Passion fruit is a native of southern Brazil and it is of two distinct forms: The forma edulis, the purple passion fruit and forma flavicarpa, the yellow passion fruit. This sweet seedy fruit is cultivated commercially in tropical and subtropical areas of the world5. The juice blends well with other fruit juices and is used in fruit salads and processed products such as ice cream, sherbet, nectar, juices, concentrate, squash, jams and jellies5. It has earlier been reported that Passion fruit could be utilized as a traditional folk medicine as well as a cosmetic moisturizing agent in several countries6. Although, a high premium has been placed on the fruit for its major use as juice and flavour in many products, other parts of the plant are also known for their medicinal values due to the bioactive phytochemical constituents it contains. Native Brazilians had used extracts of the leaves and flowers to treat asthma, bronchitis, whooping cough, urinary infections and as sedatives7. The nutritional value of any plant-based diet depends on the nutritional and antinutritional contents of the plant. The plant-based diet contains antinutrients that limit the bioavailability of nutrients such as minerals, vitamins, carbohydrates and proteins in the human body. Although, these antinutrients serve as a natural defense against infections in plants, they bind to micronutrients and inhibit their absorption in the human body during digestion preventing optimum utilization. Antinutrients become beneficial if a specific, homogenous dietary plan consisting majorly of uncooked meals is avoided8. It has been reported that antioxidant and free radical scavenging activities of the extracts from different parts of the P. edulis plant have been studied via ABTS9. The leaves of the Passion fruit plant was considered as good resources of minerals like calcium and zinc due to the high contents of both minerals in the plant. The contents of N, P, K and Zn are comparatively high while the Ca, Mg, B, Cl and Mn are moderately low in the youngest leaves10.
This study however seeks to evaluate the nutritional as well as antinutritional components, vitamin C and mineral contents, as well as in vitro antioxidant potentials of various parts of the plant.
MATERIALS AND METHODS
Study area: This study was designed and carried out in the Department of Medical Biochemistry, Ekiti State University, Ado-Ekiti, Nigeria between October, 2018 and August, 2019.
Sample collection: Fresh samples of the plant passion fruit (Passiflora edulis Sims) were obtained from Ifaki-Ekiti and was identified and authenticated by the Chief Technologist of the Department of Plant Science and Biotechnology of Ekiti State University, Ado-Ekiti, Nigeria and the plant specimen were preserved with Herbarium number of (UHAE 2020052). The Passiflora edulis plant was separated into leaf, leaf stalk, fruit pod, fruit seed and fruit juice. The leaf, leaf stalk, fruit pod and fruit seed were air-dried, crushed, and subsequently ground to powder separately with a Marlex Excella laboratory blender.
Proximate analysis was carried out on the dried powdered plant leaves according to the procedure of the Association of Official Analytical Chemists11. The percentages of moisture content, ash content, crude fibre, crude protein, crude fat and carbohydrate were determined and calculated accordingly. The total carbohydrate in the sample was obtained by difference. The amount of the percentage moisture, ash, crude fat, crude protein and crude fibre was subtracted from 10012:
The mineral elements composition was determined from the ash obtained during proximate analysis which was digested and dissolved with dilute hydrochloric acid solution using Atomic Absorption Spectrophotometer (Buck Scientific, East Norwalk, CT, United States of America) and flame Photometer (FP 202 PG).
The vitamin C content of the various parts of the plant was determined using modified UV-visible spectrophotometer method described by AL Majidi et al.13. About 10 gm of each blended and the filtered sample was transferred into a 100 mL volumetric flask, homogenized by using 50 mL acetic acid solution with shaken, 4-5 drops of bromine water have been added until the solution became coloured, then a few drops of thiourea solution were added to it to remove the excess bromine and thus the clear solution was obtained. Then the solution of 2, 4-dinitrophenyl hydrazine was added thoroughly to the various sample solutions and separately as well as the various standard solutions and the blank. Then, the solutions were all made up to the 100 mL mark with acetic acid and the absorbance of the resulting solutions were measured using a UV-visible spectrophotometer at 520 nm to determine the concentrations of ascorbic acid in the various samples from the standard calibration curve.
The antinutrients analyses of phytate, oxalate and tannin were done according to the methods reported by Emmanuel and Deborah14.
The in vitro antioxidant analyses of 1,1-diphenyl-2-picryhydrazyl (DPPH) free radical scavenging ability; 2, 2’-azino-bis (3-ethylbenthiazoline-6-sulphonic acid) (ABTS) scavenging ability; nitric oxide (NO) radical scavenging ability; ferric reducing antioxidant power; total phenolic and flavonoids contents in the plant samples were evaluated as described below:
• |
1,1-diphenyl-2-picryhydrazyl (DPPH) free radical scavenging ability of the extract was determined using the modified method of Rahman et al.15 |
|
• |
Briefly, 1.0 mL of different concentrations (20, 40 and 80 mg mL–1) of the extracts were placed in respective test tubes. 1.0 mL of 0.1 mM methanolic DPPH solution was added to the samples. These samples were vortexed and incubated in dark at room temperature for 30 min. The respective solutions were thoroughly mixed and incubated in the dark for 30 min before absorbance was measured at 516 nm. Decreased absorbance of the sample indicates DPPH free radical scavenging capability. Distilled water was replaced for the extract in the control. Percentage radical scavenging ability was calculated using the following expression: |
Determination of ABTS free radical scavenging ability: The 2, 2’-azino-bis (3-ethylbenthiazoline-6-sulphonic acid) (ABTS) scavenging ability was determined using the modified method of Lalhminghlui and Jagetia16. ABTS aqueous solution with K2S2O8 (2.45 mM L–1 final conc.) in the dark for 16 hrs and adjusting the absorbance at 734 nm to 0.700 with ethanol. To 0.2 mL of various concentrations of the extract or standard, 1.0 mL of distilled DMSO and 0.16 mL of 2, 2'-azino-bis (3-ethylbenthiazoline-6-sulphonic acid solution were added and incubated for 20 min after which absorbance of the solutions was measured spectrophotometrically at 734 nm. The ABTS scavenging antioxidant capacity was subsequently calculated using the standard of (Trolox, BHA, BHT or ascorbic acid).
Determination of nitric oxide (NO) radical scavenging ability: The modified methods of Jagetia and Baliga17 was used to determine the nitric oxide radical scavenging ability. Sodium Nitroprusside in aqueous solution at physiological pH 7.0 spontaneously generates NO, which interacts with oxygen to produce nitrite ions that can be estimated by use of Greiss reagent [1.0 mL sulfanilic acid reagent (0.33% prepared in 20% glacial acetic acid at room temperature for 5 min with 1 mL of naphthyl ethylenediamine dichloride (0.1% w/v)].
Determination of ferric reducing antioxidant power: The reducing property of the extract was determined by the modified method18. This method is based on the reduction of (Fe3+) ferricyanide in stoichiometric excess relative to the antioxidants. Different concentrations of the methanolic extract of the sample and its various fractions (10-50 μg mL–1) were added to 1.0 mL of 200 mM of sodium phosphate buffer pH 6.6 and 1.0 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50oC for 20 min, thereafter 1.0 mL of freshly prepared 10% TCA was quickly added and centrifuged at 2000 rpm for 10 min, 1.0 mL of the supernatant was mixed with 1.0 mL of distilled water and 0.25 mL of 0.1% of FeCl3 solution was added. Distilled water was used for blank without the test sample while the control solution contained all other reagents except the 0.1% potassium ferricyanide. Absorbances of these mixtures were measured at 700 nm using a spectrophotometer. Decreased absorbance indicates the ferric reducing power capability of the sample:
| • | Percentage ferric reducing antioxidant power (%) was subsequently calculated: |
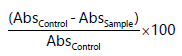 |
Estimation of total phenolic content: The extractable phenol content was determined on the extracts using the method reported by Al-Ameri et al.19. About 0.2 mL of the extract was mixed with 1.5 mL of 10% Folin ciocalteau’s reagent and 2 mL of 7.5% Sodium carbonate. The reaction mixture will be subsequently incubated at 45°C for 40 min and the absorbance was measured at 700 nm in the spectrophotometer, garlic acid would be used as standard phenol. The concentration of total phenolic compounds in the extract was determined by using the formula: Total phenolic contents were expressed in terms of gallic acid equivalent, GAE (standard curve equation):
Total flavonoid determination: The total flavonoid composition of the extract was assayed with the aid of a colourimeter as developed20. About 0.2 mL of the extract was added to 0.3 mL of 5% NaNO3 at zero time. After 5 min, 0.6 mL of 10% AlCl3 was added and after 6 mins, 2 mL of 1M NaOH solution was added to the mixture followed by the addition of 2.1 mL of distilled water. The absorbance of the solution was read at 510 nm against the reagent blank and flavonoid content was stated as mg garlic acid equivalent.
Statistical analysis: The results were expressed as Mean±Standard error of the mean of three determinations.
RESULTS AND DISCUSSION
The proximate analysis results shown in Table 1 reveal that the leaves are a very good source of carbohydrates and proteins with the lowest amount of dietary fats. Its ash content also adds up to the fact that it is a reservoir of minerals needed for essential body functioning and development21. The leaves were found to contain the lowest amount of moisture content which reveals its stability and longer shelf life on storage as moisture content is an indication of the stability of any food to spoilage by microbial action22,23. Its low-fat content shows that consumption of the leaves could help in the prevention of cardiovascular disorders associated with the consumption of food high in fats.
| Table 1: | Proximate composition of various parts of Passiflora edulis plant | |||
| Parts of plant | Dry matter (%) |
C. fiber (%) |
Ash (%) |
C. protein (%) |
C. fat (%) |
Moisture (%) |
CHO (%) |
| Leaves | 84.00±0.24 |
8.81±0.560 |
15.59±0.04 |
2.64±0.00 |
0.73±0.05 |
3.57±0.07 |
68.66±0.60 |
| Leaf stalk | 77.39±1.04 |
34.49±0.33 |
9.39±0.330 |
1.19±0.01 |
3.83±0.12 |
6.19±0.17 |
44.90±0.68 |
| Fruit pod | 83.88±0.21 |
16.60±0.37 |
12.11±0.49 |
0.98±0.00 |
2.24±0.27 |
6.66±0.17 |
61.40±0.52 |
| Fruit seed | 59.35±0.87 |
34.11±0.69 |
3.72±0.130 |
1.46±0.01 |
14.36±0.30 |
5.64±0.21 |
40.71±0.41 |
| Fruit juice | - |
- |
0.14±0.000 |
1.78±0.24 |
- |
98.07±0.17 |
- |
The leaf stalk and seeds were determined to be high in fats and fibre. The high-fat content of these parts of the plant analyzed reveals that they are good sources of phospholipids and sterols which are structural building units of biological membranes in living cells24. According to Antia et al.25, food rich in fats increases palatability and may increase appetite. Their rich fibre content could aid constipation and reduce the risks of certain diseases such as colon cancer, diabetes, coronary heart disease26-28. Fibres also aid in the dilution of increased colonic bile associated with consumption of fat-rich food29.
Table 2 shows the antinutrient concentrations of phytate, oxalate and tannin in the Passiflora edulis plant parts. Antinutrients generally play biological roles in plants and animals. Phytate forms insoluble complexes with minerals like magnesium, calcium, zinc, iron and copper preventing optimum exploitation of these minerals by interfering with their absorption30. It also impairs the digestion of carbohydrates, proteins and inhibits enzymes activity. It has been reported that consuming a diet consisting of 10-60 mg/100 g phytate over a long period may result in a mineral deficiency31. Phytate content was found to be highest in fruit pod followed by the fruit seed than any other parts of the plant as shown in the Table 2. The reported concentrations of phytate in all the parts of the plants however fall below the maximum acceptable concentration in the human diet.
Most antinutrients like oxalates once ingested cannot be removed from the urinary tracts while high intake of oxalate may cause accumulation of kidney stones, weakness and abdominal pains32. The oxalates contents of all parts analyzed were well below the accepted daily intake amounts of 50-60 mg day–1 recommended for kidney stone patients33. The tannin contents of all parts analyzed were well below the maximum acceptable daily increase in the human diet as reported by Fekadu et al.34, hence its antinutritional effect in the consumption of all parts of the plants analyzed are insignificant. Tannin, a water-soluble polyphenol is found in varying amounts in many edible fruits and vegetables. Although, tannin binds to protein and reduces protein digestibility, it has been shown that tannin exhibits potent antiviral, anti-parasitic, anti-bacterial, antioxidant and radical scavenging potentials hence daily diet containing tannin may help to prevent many types of diseases35-37.
The vitamin C contents are well distributed in all the various parts of the Passiflora edulis plant as shown in Table 3. The leaves and the seed contained higher levels of vitamin C when compared to the leaf stalk, pod and juice.
Vitamin C is essential to the body and plays a prominent role as a powerful antioxidant38, Vitamin C assists in converting iron that is poorly absorbed, such as plant-based sources of iron, into a form that is easier to absorb39. Many plants and plants products have been reported to contain vitamin C which is of both nutritional and medicinal importance as reported in previous studies by researchers40,41.
The in vitro antioxidant parameters were investigated on the various parts of the plant of passion fruits to evaluate their distribution as seen in Table 4. The ferric reducing antioxidant power of different parts of the plants showed the highest concentration in the order of leaves, seed, pod, stalk and juice also in concentration-dependent format. Antioxidant compounds cause the reduction of ferric (Fe3+) form to the ferrous (Fe2+) form because of their reductive capabilities. The lower the value of ferric reducing antioxidant power, the lower the antioxidant property of the plant while the higher the value of ferric reducing antioxidant power, the higher the antioxidant property. The ferric reducing antioxidant power of some plants have been reported by researchers42,43.
| Table 2: | Some antinutrient contents of various parts of Passiflora edulis plant | |||
| Parts of plant | Phytate (mg g–1) |
Oxalate (mg g–1) |
Tannin (mg g–1) |
| Leaves | 8.65±0.41 |
0.63±0.00 |
2.23±0.03 |
| Leaf stalk | 4.53±0.41 |
0.81±0.00 |
2.50±0.01 |
| Fruit pod | 12.36±0.00 |
0.49±0.04 |
4.14±0.00 |
| Fruit seed | 11.12±0.41 |
0.67±0.04 |
4.19±0.01 |
| Fruit juice | 6.59±0.00 |
0.63±0.00 |
2.87±0.01 |
| Table 3: | Vitamin C contents of various parts of Passiflora edulis plant (mg g–1) | |||
| Parts of plant | Vitamin contents (mg g–1) |
| Juice | 21.70±0.20 |
| Seed | 62.26±0.17 |
| Pod | 50.89±0.11 |
| Leaves | 62.77±0.17 |
| Leaf stalk | 52.67±0.53 |
| Table 4: | Some in vitro antioxidant analyses of various parts of Passiflora edulis plant | |||
| Parts of plant | Concentration (mg mL–1) |
FRAP (mg g–1) (mg mL–1) |
DPPH (%) |
ABTS (mMol g–1) |
Fe2+chelation (%) |
Total phenol(mg g–1) |
Flavoniod (mg g–1) |
| Leaves | 50 |
2.90±0.03 |
59.41±0.11 |
0.01±0.00 |
20.08±0.25 |
8.39±0.04 |
0.36±0.00 |
75 |
5.29±0.03 |
66.85±0.11 |
0.02±0.00 |
35.74±0.25 |
18.05±0.04 |
0.49±0.00 |
|
100 |
8.78±0.03 |
75.53±0.11 |
0.02±0.00 |
50.67±0.25 |
25.27±0.04 |
1.08±0.00 |
|
| Stalk | 50 |
2.00±0.03 |
39.03±0.11 |
0.01±0.00 |
2.97±0.25 |
5.86±0.04 |
0.10±0.00 |
75 |
3.81±0.03 |
43.55±0.11 |
0.01±0.00 |
20.93±0.25 |
11.68±0.04 |
0.23±0.00 |
|
100 |
6.34±0.03 |
57.80±0.11 |
0.02±0.00 |
41.44±0.25 |
18.59±0.04 |
0.34±0.00 |
|
| Pod | 50 |
2.72±0.03 |
44.12±0.11 |
0.01±0.00 |
18.14±0.25 |
6.52±0.04 |
0.12±0.00 |
75 |
4.84±0.03 |
52.34±0.11 |
0.01±0.00 |
27.73±0.25 |
12.29±0.04 |
0.27±0.00 |
|
100 |
8.34±0.03 |
62.69±0.11 |
0.02±0.00 |
42.17±0.25 |
20.41±0.04 |
0.36±0.00 |
|
| Seed | 50 |
2.80±0.03 |
23.06±0.11 |
0.01±0.00 |
13.89±0.25 |
8.03±0.04 |
0.16±0.00 |
75 |
5.06±0.03 |
54.37±0.11 |
0.01±0.00 |
30.76±0.25 |
15.61±0.04 |
0.39±0.00 |
|
100 |
8.48±0.03 |
65.49±0.11 |
0.02±0.00 |
48.24±0.25 |
23.94±0.04 |
0.53±0.00 |
|
| Juice | 50 |
1.35±0.03 |
11.52±0.11 |
0.01±0.00 |
1.27±0.25 |
1.96±0.04 |
0.02±0.00 |
75 |
2.53±0.03 |
15.21±0.11 |
0.01±0.00 |
9.77±0.25 |
3.56±0.04 |
0.07±0.00 |
|
100 |
4.32±0.03 |
21.39±0.11 |
0.01±0.00 |
25.55±0.25 |
5.64±0.04 |
0.11±0.00 |
The 1,1-diphenyl-2-picryhydrazyl (DPPH) radical scavenging antioxidant potential of the various parts of the plants were determined as shown in Table 4. The ability of any plant extract to scavenge DPPH radicals also expresses antioxidant potentials. The leaf and the pod showed higher antioxidant potentials than other parts though all the parts possessed antioxidant properties. The observation on DPPH scavenging ability by the various parts of the Passiflora edulis recorded in this study corroborated the earlier reported studies on different plants and plant parts by researchers44-46. In their studies, different plants and plants parts possessed. The ABTS radical scavenging capacity of the Passiflora edulis plant parts was also investigated as seen in Table 4. The results obtained showed all plant parts possessed ABTS radical scavenging ability in mMol g–1. The ability of any plant or plant product to scavenge free radicals is also an indicator of its antioxidant ability. All the parts of the plant under study possessed iron chelation as observed in the table of results above. Iron chelation is the ability of plant or plant product extract to remove excess or reduce iron overload from the body which had been reported to be an antioxidant process. It has also been reported that iron chelator does not only reduce excess iron but also scavenge reactive oxygen species (ROS)47. Table 4 also showed that the plant's parts contained both flavonoids and total phenolic compounds which are powerful antioxidants. The presence of all these antioxidant potentials in this plant and its various parts is an indication of the medicinal property of the various parts.
| Table 5: | Some nutritionally viable elemental composition of various parts of Passiflora edulis plant (mg kg–1) | |||
| Parts of plant | Mn |
Na |
Ca |
Cr |
Pb |
Zn |
Ni |
Fe |
Cd |
Cu |
Co |
| Leaves | 1.82 |
4.6 |
494 |
ND |
0.98 |
89.58 |
14.77 |
256.92 |
ND |
13.71 |
0.98 |
| Leaf stalk | 0.32 |
3.16 |
194 |
ND |
8.95 |
45.76 |
10.94 |
91.52 |
ND |
9.95 |
ND |
| Fruit pod | 0.12 |
1.94 |
20.48 |
ND |
1.99 |
63.63 |
13.92 |
112.35 |
ND |
10.94 |
ND |
| Fruit seed | 0.12 |
1.14 |
5.84 |
ND |
7.83 |
61.64 |
21.53 |
121.33 |
ND |
11.74 |
0.98 |
| Fruit juice (mg L–1) | 0.04 |
1.46 |
5.62 |
ND |
0.06 |
1.82 |
0.36 |
8.32 |
ND |
0.18 |
0.04 |
The analysis of the mineral content in Table 5 showed that the leaves are a good source of most ions analyzed especially Ca and Fe which indicates that the inclusion of the leaves in daily diet would be effective against bone loss as well as the requirement for coagulation of the blood48. The seed which is also rich in Fe could be recommended for eaten along with the fruit and leaves as Fe improves resistance to infection and aids haemoglobin formation. Cobalt showed the least ion concentration for the plant parts analyzed while chromium and cadmium were not detected. The fruit juice showed the least concentration of all minerals analyzed.
CONCLUSION
Passiflora edulis plant is a veritable and delightful plant from the observations in this study. The results obtained showed that the various parts of Passiflora edulis plant contained powerful antioxidant potentials, significant vitamin C content, useful nutritional composition and nutritional valuable minerals but fewer antinutrient factors. This has led credence to possibilities of its applications as a source of therapeutic medicine to humans and usefulness in the animal foods and feed composition.
SIGNIFICANCE STATEMENT
This study discovers the possible utilizations of one if not all the parts of the plant for therapeutics and nutritional supplementations that can be beneficial for prevention and treatments of diseases like cancer, diabetics and other degenerative diseases are rampant especially in the third world countries where the access to orthodox medicine is limited. This study will help in identifying potential tropical plants of medicinal and nutritional importance that many researchers were not able to explore. Thus, new drugs for various diseases on drug development may be arrived at.
REFERENCES
- Dhawan, K., S. Dhawan and A. Sharma, 2004. Passiflora: A review update. J. Ethnopharmacol., 94: 1-23.
- Zhang, J., R. Koike, A. Yamamoto, M. Ukiya and M. Fukatsu et al., 2013. Glycosidic inhibitors of melanogenesis from leaves of Passiflora edulis. Chem. Biodivers., 10: 1851-1865.
- Yuan, T.Z., C.L. Kao, W.J. Li, H.T. Li and C.Y. Chen et al., 2017. Chemical constituents of leaves of Passiflora edulis. Chem. Nat. Compd., 53: 1165-1166.
- Hu, Y., L. Jiao, M.H. Jiang, S. Yin and P. Dong et al., 2018. A new C-glycosyl flavone and a new neolignan glycoside from Passiflora edulis Sims peel. Nat. Prod. Res., 32: 2312-2318.
- Zeraik, M.L., D. Serteyn, G. Deby-Dupont, J.N. Wauters and M. Tits et al., 2011. Evaluation of the antioxidant activity of passion fruit (Passiflora edulis and Passiflora alata) extracts on stimulated neutrophils and myeloperoxidase activity assays. Food Chem., 128: 259-265.
- Xu, F.Q., N. Wang, W.W. Fan, C.T. Zi and H.S. Zhao et al., 2016. Protective effects of cycloartane triterpenoides from Passiflora edulis Sims against glutamate-induced neurotoxicity in PC12 cell. Fitoterapia, 115: 122-127.
- Zibadi, S. and R.R. Watson, 2004. Passion fruit (Passiflora edulis). Evidence-Based Integr. Med., 1: 183-187.
- Popova, A. and D. Mihaylova, 2019. Antinutrients in plant-based foods: A review. Open Biotechnol. J., 13: 68-76.
- Zas, P., S. John, 2017. Phytochemical investigation and antioxidant activities of Passiflora edulis (passion fruit) leaves from Ukhrul District, Manipur, India. World J. Pharm. Res., 6: 793-801.
- Freitas, M.S.M., P.H. Monnerat, I.J.C. Vieira and A.J. Cordeiro de Carvalho, 2007. Flavonoids and mineral composition the leaf in yellow passion fruit plant in function of leaves at positions in the branch. Ciênc. Rural, 37: 1634-1639.
- Syad, A.N., K.P. Shunmugiah and P.D. Kasi, 2013. Seaweeds as nutritional supplements: Analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomed. Preventive Nutr., 3: 139-144.
- Ramdath, D.D., Zhan-Hui Lu, P.L. Maharaj, J. Winberg and Y. Brummer et al., 2020. Proximate analysis and nutritional evaluation of twenty Canadian lentils by principal component and cluster analyses. Foods. 9.
- AL Majidi, M.I.H. and H.Y. ALQubury, 2016. Determination of Vitamin C (ascorbic acid) contents in various fruit and vegetable by UV-spectrophotometry and titration methods. J. Chem. Pharm. Sci., 9: 2972-2974.
- Emmanuel, E. and S. Deborah, 2018. Phytochemical and anti-nutritional studies on some commonly consumed fruits in Lokoja, Kogi State of Nigeria. Gen. Med. Open. 2.
- Rahman, M.M., M.B. Islam, M. Biswas and A.H.M.K. Alam, 2015. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 8.
- Lalhminghlui, K., G.C. Jagetia, 2018. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA. 4.
- Jagetia, G.C. and M.S. Baliga, 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. J. Med. Food, 7: 343-348.
- Pulido, R., L. Bravo and F. Saura-Calixto, 2000. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem., 48: 3396-3402.
- Al-Ameri, S.A.H. and M.M.A. Al-Shemari, 2014. Determination the total phenolic contents in some foods using solid phase extraction. Al-Nahrain J. Sci., 17: 38-45.
- Bao, J., Y. Cai, M. Sun, G. Wang and H. Corke, 2005. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem., 53: 2327-2332.
- Haque, N., A. Hughes, S. Lim and C. Vernon, 2014. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources, 3: 614-635.
- N’zebo, N. Jean-Michel, A.P. Ahi, K.M. Dje, A.F. Kabran and L.P. Kouamé et al., 2019. Chemical composition and mineral bioavailability of Tetrapleura tetraptera (Schumach & Thonn.) Taub. fruit pulp consumed as spice in South-Eastern Côte d’Ivoire. Turk. J. Agric. Food Sci. Technol., 7: 1817-1824.
- Nwofia, G.E., N.N. Victoria and K.N. Blessing, 2012. Nutritional variation in fruits and seeds of pumpkins (Cucurbita spp) accessions from Nigeria. Pak. J. Nutr., 11: 946-956.
- van Meer, G., D.R. Voelker and G.W. Feigenson, 2008. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol., 9: 112-124.
- Antia, B.S., E.J. Akpan, P.A. Okon and I.U. Umoren, 2006. Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pak. J. Nutr., 5: 166-168.
- Jimam, N.S., K.D. Christopher and U.O. Cynthia, 2015. Nutritional and antinutritional analysis of Sesamum radiatum leaves. World J. Pharm. Sci., 3: 1516-1519.
- Dawczynski, C., R. Schubert and G. Jahreis, 2007. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem., 103: 891-899.
- Okaraonye, C.C. and J.C. Ikewuchi, 2009. Nutritional and antinutritional components of Pennisetum purpureum (Schumach). Pak. J. Nutr., 8: 32-34.
- Zhao, L., F. Zhang, X. Ding, G. Wu and Y.Y. Lam et al., 2018. Gut bacteria selectively promoted by dietary fibers alle-viate type 2 diabetes. Science, 359: 1151-1156.
- Frontela, C., J.F. Haro, G. Ros and C. Martinez, 2008. Effect of dephytinization and follow-on formula addition on in vitro iron, calcium, and zinc availability from infant cereals. J. Agric. Food Chem., 56: 3805-3811.
- Elinge, C.M., A. Muhammad, F.A. Atiku, A.U. Itodo, I.J. Peni, O.M. Sanni and A.N. Mbongo, 2012. Proximate, mineral and anti-nutrient composition of pumpkin (Cucurbitapepo L) seeds extract. Int. J. Plant Res., 2: 146-150.
- Olawoye, B.T., S.O. Gbadamosi and F. Yildiz, 2017. Effect of different treatments on in vitro protein digestibility, antinutrients, antioxidant properties and mineral composition of Amaranthus viridis seed. Cogent Food Agric. 3.
- Massey, L.K., R.G. Palmer and H.T. Horner, 2001. Oxalate content of soybean seeds (Glycine max: Leguminosae), soyfoods, and other edible legumes. J. Agric. Food Chem., 49: 4262-4266.
- Fekadu, H., F. Beyene and G. Desse, 2013. Effect of traditional processing methods on nutritional composition and anti-nutritional factors of Anchote (Coccinia abyssinica (Lam.) Cogn) tubers grown in Western Ethiopia. J. Food Process. Technol., 4: 1-8.
- Delimont, N.M., M.D. Haub and B.L. Lindshield, 2017. The impact of tannin consumption on iron bioavailability and status: A narrative review. Curr. Dev. Nutr., 1: 1-12.
- Adeyemo, S.M. and A.A. Onilude, 2013. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Food J., 31: 84-90.
- Akiyama, H., K. Fujii, O. Yamasaki, T. Oono and K. Iwatsuki, 2001. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother., 48: 487-491.
- Alessio, H.M., A.H. Goldfarb and G. Cao, 1997. Exercise-induced oxidative stress before and after vitamin C supplementation. Int. J. Sport Nutr. Exercise Metab., 7: 1-9.
- Hurrell, R. and I. Egli, 2010. Iron bioavailability and dietary reference values. Am. J. Clin. Nut., 91: 1461S-1467S.
- Rivelli, A.R., M.C. Caruso, S. de Maria and F. Galgano, 2017. Vitamin C content in leaves and roots of horseradish (Armoracia rusticana): Seasonal variation in fresh tissues and retention as affected by storage conditions. Emir. J. Food Agric., 29: 799-806.
- Ogunlesi, M., W. Okiei, L. Azeez, V. Obakachi, M. Osunsanmi and G. Nkenchor, 2010. Vitamin C contents of tropical vegetables and foods determined by voltammetric and titrimetric methods and their relevance to the medicinal uses of the plants. Int. J. Electrochem. Sci., 5: 105-115.
- Gohari, A.R., H. Hajimehdipoor, S. Saeidnia, Y. Ajani and A. Hadjiakhoondi, 2011. Antioxidant activity of some medicinal species using FRAP assay. J. Med. Plants, 10: 54-60.
- Al-Laith, A.A., J. Alkhuzai and A. Freije, 2019. Assessment of antioxidant activities of three wild medicinal plants from Bahrain. Arabian J. Chem., 12: 2365-2371.
- Mahdi-Pour, B., S.L. Jothy, L.Y. Latha, Y. Chen and S. Sasidharan, 2012. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac. J. Trop. Biomed., 2: 960-965.
- Guimarães, S.F., I.M. Lima and L.V. Modolo, 2020. Phenolic content and antioxidant activity of parts of Passiflora edulis as a function of plant developmental stage. Acta Bot. Brasilica, 34: 74-82.
- Saeed, N., M.R. Khan and M. Shabbir, 2012. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary Altern. Med.. 12.
- Adjimani, J.P. and P. Asare, 2015. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep., 2: 721-728.
- Sunyecz, J.A., 2008. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manage., 4: 827-836.
How to Cite this paper?
APA-7 Style
Abass,
O.O., Doris,
S., Olorunshola,
A.T., Festus,
I. (2024). Chemical and Some Biochemical Assessments of Various Parts of Passion Fruit (Passiflora edulis Sims) Plant. Asian Journal of Biological Sciences, 17(1), 32-40. https://doi.org/10.3923/ajbs.2024.32.40
ACS Style
Abass,
O.O.; Doris,
S.; Olorunshola,
A.T.; Festus,
I. Chemical and Some Biochemical Assessments of Various Parts of Passion Fruit (Passiflora edulis Sims) Plant. Asian J. Biol. Sci 2024, 17, 32-40. https://doi.org/10.3923/ajbs.2024.32.40
AMA Style
Abass
OO, Doris
S, Olorunshola
AT, Festus
I. Chemical and Some Biochemical Assessments of Various Parts of Passion Fruit (Passiflora edulis Sims) Plant. Asian Journal of Biological Sciences. 2024; 17(1): 32-40. https://doi.org/10.3923/ajbs.2024.32.40
Chicago/Turabian Style
Abass, Oseni, Olatunda, Seyinde Doris, Abe Taiwo Olorunshola, and Igbe Festus.
2024. "Chemical and Some Biochemical Assessments of Various Parts of Passion Fruit (Passiflora edulis Sims) Plant" Asian Journal of Biological Sciences 17, no. 1: 32-40. https://doi.org/10.3923/ajbs.2024.32.40

This work is licensed under a Creative Commons Attribution 4.0 International License.



