Comparative Anti-Oxidative and Acetylcholinesterase Inhibition Potentials of Selected Plants in Treating Alzheimer’s and Degenerative Diseases
| Received 30 Jul, 2023 |
Accepted 22 Dec, 2023 |
Published 31 Mar, 2024 |
Background and Objective: Acetylcholinesterase inhibitors are important therapeutic agents in Alzheimer’s and other cognitive complications. The study aimed to evaluate acetylcholinesterase inhibition, antioxidant activity and other biochemical indices of the three solvent-extracts of five plant species from South-Western Nigeria. Materials and Methods: The leaves were extracted with solvents, phytochemical screening and in vitro antioxidant properties of the extracts were determined. Rats weighing between 140 to 150 g were divided into groups comprising of normal control, 2,2-dichlorovinyl dimethyl phosphate (DDVP), aqueous, methanol and ethanol groups for each of the five plants. The rats were induced with 3.3 mg kg–1 b.wt., of DDVP for two weeks with the aim of inducing cognitive complications while the induced animals were treated with 3.3 mg kg–1 b.wt., of the extracts of the five plant species for another two weeks. The rats were anaesthetized and quickly dissected individually to collect serum and organs homogenates to evaluate the studied parameters using standard procedures. Results: The three extracts of the five plants show powerful in vitro and in vivo antioxidant properties, impressive anti-lipid peroxidation potentials and promising acetylcholinesterase inhibition activity. Conclusion: These plant solvents extracts could be locally utilized in drug developments to prevent and cure the consequences of Alzheimer disease (AD) and associated disorders.
| Copyright © 2024 Oseni and Adeleye. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Alzheimer’s disease is characterized by selective neuronal cell death, the presence of extra cellular amyloid deposits in the core of neurotic plaques and the formation of intra neuronal neurofibrillary tangles in the brain of afflicted individuals1. According to the cholinergic hypothesis, the inhibition of acetylcholinesterase (AChE), an enzyme that catalyzes acetylcholine hydrolysis, increases the levels of acetylcholine in the brain, thus improving cholinergic functions in AD patients2. The cholinergic hypothesis states that decreased cholinergic transmission plays a major role in the expression of cognitive, functional and possibly behavioral symptoms in AD3,4. The cholinergic hypothesis rests on pathological, biochemical and pharmacological observations. Oxidative stress is also implicated in the development of many neurodegenerative diseases including Parkinson’s disease (PD), Huntington’s disease, amyotrophic lateral sclerosis and Alzheimer’s disease (AD)5. One of the important strategies for treating of AD is to maintain the levels5 of acetylcholine through the inhibition of acetylcholinesterase (AChE)6. It has been noted that acetylcholinesterase enzyme (AChE) could be an striking target for the cogent drug strategy and for the detection of mechanism-based inhibitors as a result of its protagonist in the hydrolysis of the neurotransmitter called acetylcholine (ACh). These AChE inhibitors are the most active approach to treat the cognitive symptoms of Alzheimer disease (AD)7,8 as well as other possible therapeutic applications in the treatment of Parkinson's disease, senile dementia and ataxia, among others Ahmad et al.9. The AChE inhibitors as eserine, tacrine, donepezil, rivastigmine and galanthamine are the only drugs currently approved for the treatment of AD; however, these drugs are known to have limitations for clinical use due to their short-half-lives and/or unfavorable side-effects10. Dichlorvos is a synthetic organic chemical used as an insecticide. Dichlorvos does not occur naturally in the environment but is manufactured by industry.
Dichlorvos is an insecticide used on crops, animals and in pest-strips. Acute (short-term) and chronic (long-term) exposures of humans to dichlorvos results in the inhibition of an enzyme, acetylcholinesterase, with neurotoxic effects including perspiration, vomiting, diarrhea, drowsiness, fatigue, headache and at high concentrations, convulsions and coma11. Since the synthetic drugs have undesirable side effects or contraindications, the World Health Organization (WHO) has recommended the evaluation of traditional plants treatments for diabetes12. Medicinal plants are widely used worldwide to address a variety of health problems. About 25 to 50% of current pharmaceuticals are derived from plants. Modern medicine today utilizes active compounds isolated from higher plants and about 80% of these active ingredients indicate a positive correlation between their modern therapeutic use and the traditional uses13. Plants are rich in a wide variety of phytochemical metabolites which are divided into two groups: Primary and secondary metabolites. Primary metabolites consist of common sugars, amino acids, proteins and chlorophylls, while secondary metabolites include glycosides, alkaloids, saponins, phenolic compounds, terpenes, steroids and anthraquinones. Secondary metabolites have shown to possess various biological effects, which provide the scientific base for the use of herbs in the traditional medicine in many ancient communities14. Medicinal plants are extracted and processed for direct consumption as herbal or traditional medicine or prepared for experimental purposes15. These plants have been claimed to be used in the treatments of diseases, but its AChE inhibition activity has not been studied so far. Hence the main objective of the present study was to evaluate AChE inhibition, antioxidant activity and other biochemical indices of the three solvent-extracts of five plant species [scent leaf, ewe efirin (Ocimum gratissimum), wind braker leaf, ewe igunnu (Polyalthia longifolia (Sonn.), pawpaw leaf, ewe ibepe (Carica papaya), African mahogamy leaf, ewe apa (Afzelia africana) and goat weed leaf, ewe imi-esu (Ageratum conyzoides)] from South-Western Nigeria.
MATERIALS AND METHODS
Study area: This study was designed and carried out in the Department of Medical Biochemistry, Ekiti State University, Ado-Ekiti, Nigeria between October, 2017 and August, 2018.
Plants samples collection, authentication and preparation: The various plants leave of Ocimum gratissimum (UHAE 2020051), Polyalthia longifolia (Sonn.) (UHAE 2020054), Carica papaya (UHAE 2020048), Afzelia africana (UHAE 2020046) and Ageratum conyzoides (UHAE2020047) were sourced from various locations in Ado-Ekiti, Ifaki-Ekiti, Iworoko-Ekiti in Ekiti State and Ibadan in Oyo State Nigeria by detaching large quantities of leaves from their various trees of the plants. The leaves were however, authenticated at Department of Plant Science, Ekiti State University, Ado-Ekiti, Nigeria and respective Herbarium numbers were obtained.
The leaves were rinsed in water to remove any form of dirt’s. The rinsed leaves were spread in a room for some days till they were completely air dried. The air-dried leaves were pulverized with an electric blender to increase the surface area and to hasten the process of extraction.
Extraction with water, methanol and ethanol: The individual powered leaves were divided and measured into three parts using standard weighing balance. As 200 g each of the powdered leaves were extracted with 500 mL each of water, methanol and ethanol inside stoppered glass bottles with continuous shaking overnight. The extracted solutions were evaporated to obtain the respective extracts. The resulting extracts were weighed and the percentage solvent extracts yields were recorded.
The flow chart for the solvent’s extraction of the ten different plants showed as follows:
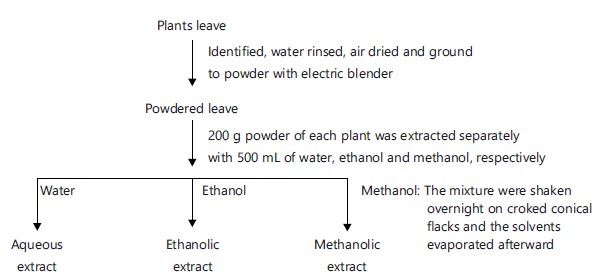 |
Preliminary phytochemical screening of the leaves’ extracts: The qualitative phytochemical screening [flavonoids, saponin, phlobatannins, terpenoids, Salkowski test for cardiac glycosides (steroidal ring or terpenoids), Keller-Killani test for cardiac glycosides (deoxysugar), Lieberman’s test for steroidal nucleus and test for tannins] of aqueous, methanol and ethanol extract fractions of the leaves were carried out according to the methods described by Trease and Evans16 and Oseni et al.17 to identify the active constituents.
In vitro antioxidants analysis of the leaves’ extracts: The quantitative in vitro scavenging activities [H2O2 (%); 2,2'-azino-bis-3-ethyl benzthiazoline-6-sulphonic acid (ABTS), nitric oxide, 2,2-diphenyl-1-picryl hydrazyl hydrate (DPPH), superoxide radicals] activities, total phenol and flavonoids contents were determined, respectively by the methods of researchers18-23.
Experimental animals: The Wistar albino rats weighing between 140 to 150 g were obtained from Animal House of College of Medicine, Ekiti State University, Ado-Ekiti, Nigeria.
Animal care and management: The 85 Wistar albino rats weighing between 140-150 g obtained from Animal House of Ekiti State University College of Medicine, Ado-Ekiti, Nigeria. They were grouped into five of five animals each. They were housed in plastic cages under standard laboratory conditions of natural light/dark cycle at room temperature and humidity. The rats were fed on standard rat pellets which were procured once from (top feeds, Nigeria) and given drinking water ad libitum. All animals were handled in accordance with the guide for the care and use of laboratory animals as detailed in the International Animal Care and Use Committee.
Experimental design and animal assay: The rats were induced with 3.3 mg kg–1 b.wt., of 2,2-dichlorovinyl dimethyl phosphate (DDVP) solution for fourteen days except group 1. The induced rats were then treated with 3.3 mg kg–1 b.wt., solution of aqueous, methanolic and ethanolic extracts of the various plants for the next fourteen days:
| Group 1: | Normal control (animals given water and rats feed ad libitum) | |
| Group 2: | DDVP induced control (animals were administered orally with 0.5 mL of 3.3 mg kg–1 b.wt., of DDVP solution) | |
| Group 3: | (3i-3v) DDVP induced animals+0.5 mL of 3.3 mg kg–1 b.wt., of aqueous solution of each extract of the plants | |
| Group 4: | (4i-4v) DDVP induced animals+0.5 mL of 3.3 mg kg–1 b.wt., of methanolic solution of each extract of the plants | |
| Group 5: | (5i-5v) DDVP induced animals+0.5 mL of 3.3 mg kg–1 b.wt., of ethanolic solution of each extract of the plants |
After the experiment, the rats in all the groups were anaesthetized with chloroform individually and quickly dissected to collect blood into the sample bottles and latter centrifuged at 3000 rpm to obtain serum, while the organs (brain, liver and heart) were removed, weighed and placed on ice-bath. While 10% of each organ homogenate was then prepared, respectively in 6.7 mM potassium phosphate buffer (pH 7.4) using the electrically top driven homogenizer.
The individual organ homogenates were centrifuged at 3,000 rpm for 10 min at 4°C to obtain clear supernatants which were stored at 8°C and used for measurement of the studied biochemical parameters.
The antioxidants enzymes activities [catalase (CAT), superoxide dismutase (SOD), glutathione-s-transferase (GST), glutathione reductase (GR) and glutathione peroxidase (GPx)], reduced glutathione (GSH) were determined by the methods described by researchers24-27. The lipid peroxidation was done by measuring the TBARS in accordance with the modified method of Garcia et al.28. The 2013 Technical Bulletin of Sigma-Aldrich Co. LLC, United States of America (www.sigmaaldrich) assay Kit was employed to determine the acetylcholinesterase activity, where one unit of AChE is the amount of enzyme that catalyzes the production of 1.0 μmol of thiocholine per min at room temperature at pH 7.5 with the aim of evaluating the inhibition of acetylcholinesterase activity. This kit has a linear range of 10-600 units L–1 of AChE activity.
Statistical analysis: The mean triplicate results were determined and represented in charts.
RESULTS
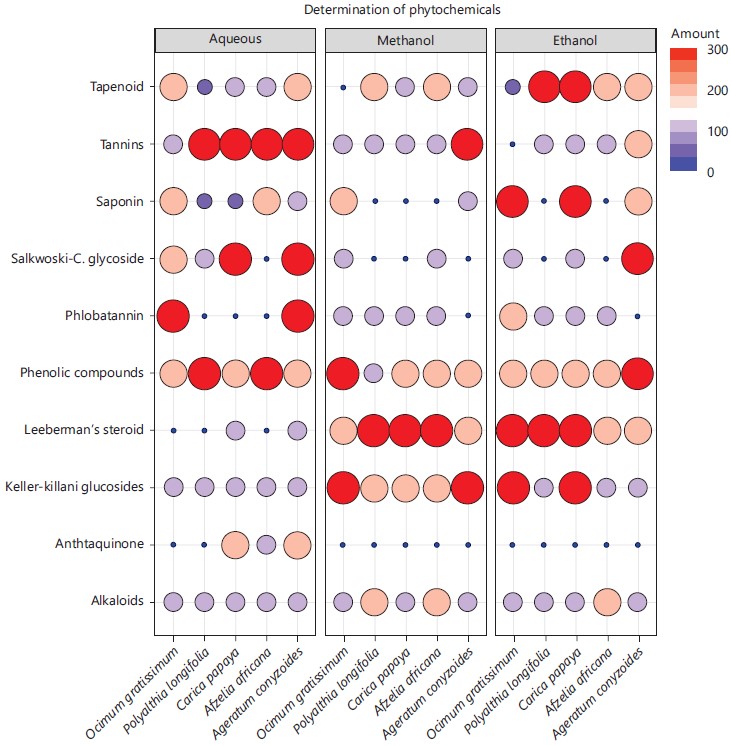
The phytochemical compositional screening can be seen in Fig. 1 showing the leaves of the five plants containing important compounds of medicinal significant at varying intensity in all the solvents extracts as studied.
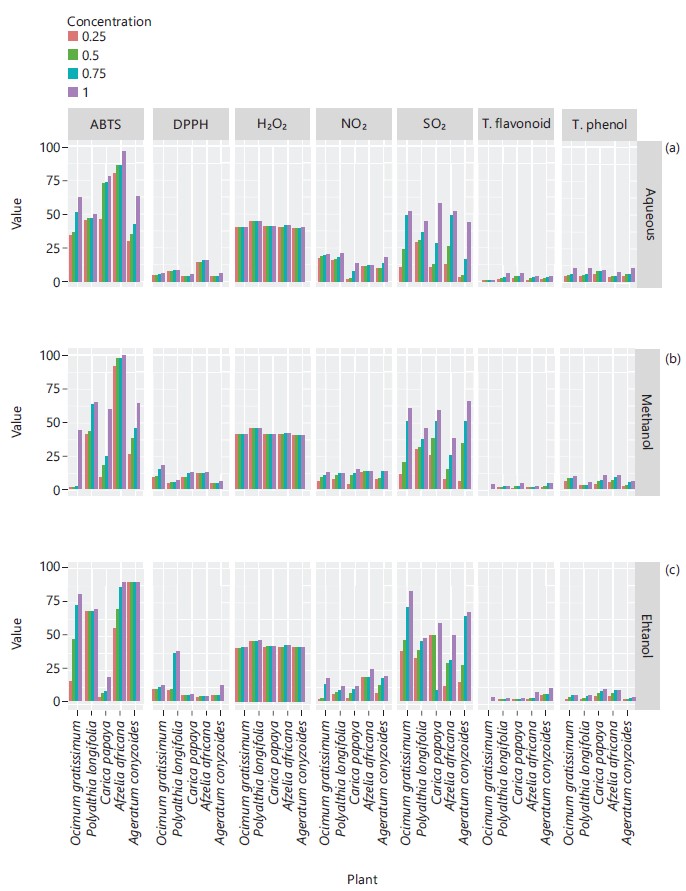
Similarly, Fig. 2 represented the (ABTS (%), DPPH (%), H2O2 (%), nitric oxide (%) and super oxide (%)) scavenging activities and concentrations of total flavonoids and total phenol of leaves extracts of plants, the scavenging activities of each plant extract increased in dose dependent manner.
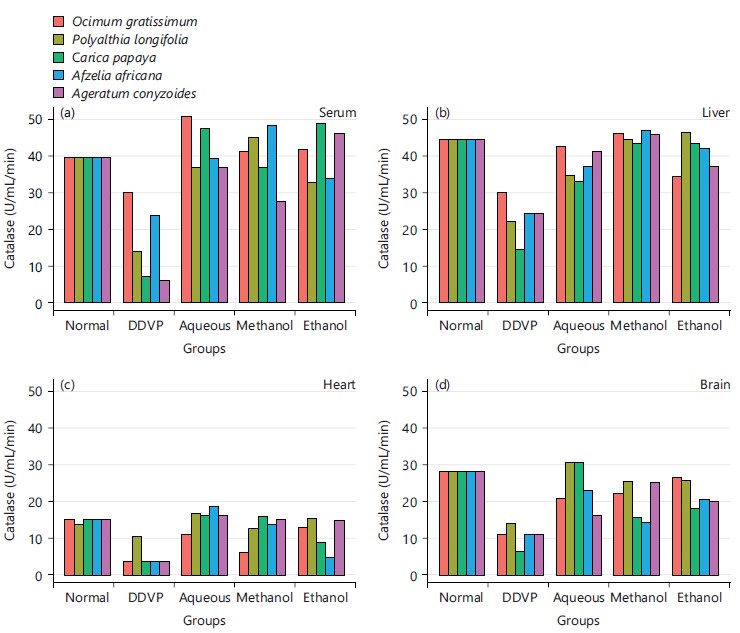
The effects of various extracts of the plants on the activity of catalase as seen in Fig. 3, the serum, liver, heart and brain tissues of induced and treated animals showing all the plants causing restored activity of the enzyme after treatments.
|
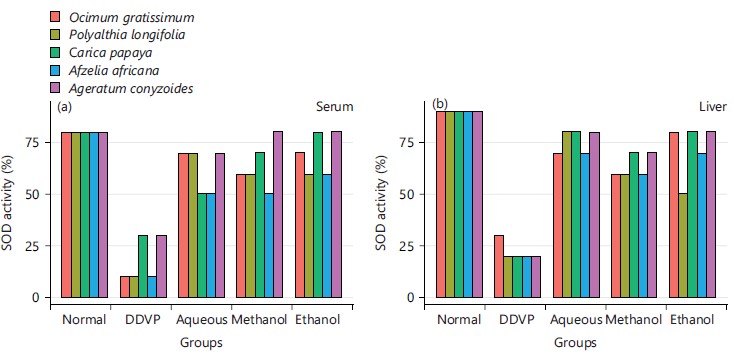
The percentage superoxide dismutase activity as presented in Fig. 4 showed the effects of various extracts of the plants indicating all the solvents extracts display increased SOD percentage activity when compared with the DDVP induced group.
The glutathione peroxidase activity of various extracts of plants from Fig. 5 showed that the extracts caused a reversal effect to normal control group when compared with the DDVP induced group.
The glutathione transferase activity in DDVP induction and treatment with various extracts of the plants was shown in Fig. 6. The extracts caused enhanced activity of the enzyme on the treated groups.
The activity of glutathione reductase in Fig. 7 revealed the improved activity on treatment with the various extracts of the plants.
|
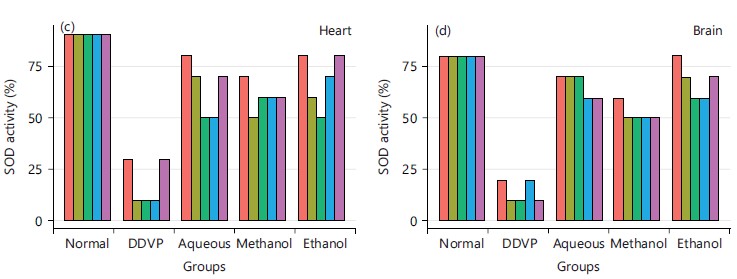
The concentration of reduced glutathione from Fig. 8 showed that the various extracts of the plants caused increased concentration of GSH in the depleted concentration of the DDVP induction group.
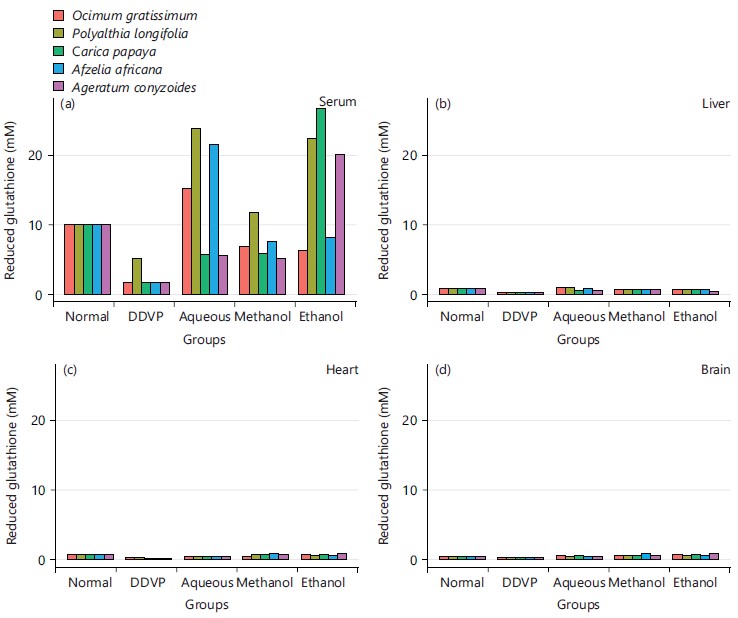
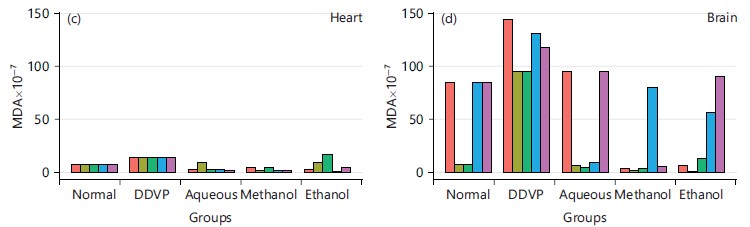
From Fig. 9, the concentration of MDA was grossly reduced from the DDVP induced group when compared to the solvent’s extracts treated groups.
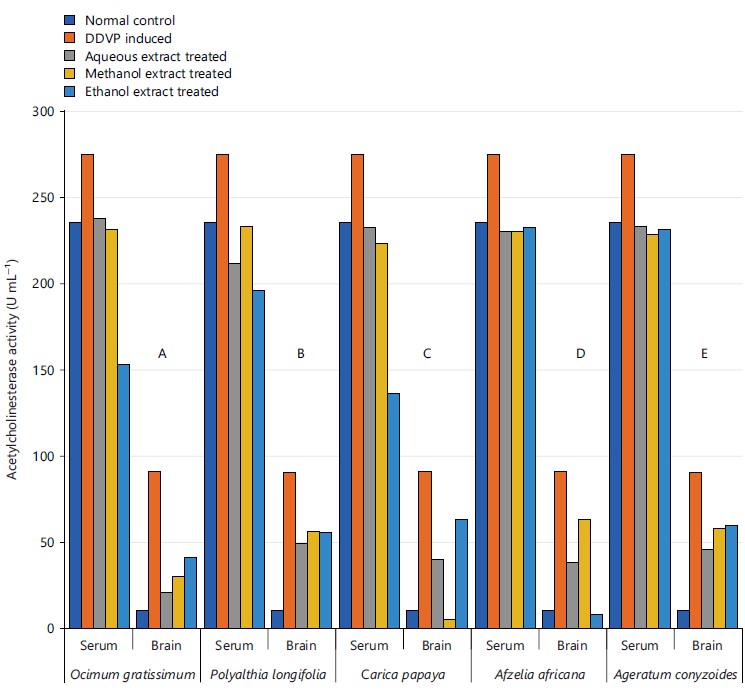
The inhibition of the acetylcholinesterase activity observed in Fig. 10 showed that the various extracts of the plants caused substantive activity inhibition from the DDVP induction group.
|
|

|
DISCUSSION
The qualitative phytochemical composition of the five plants leaves solvents extracts were evaluated to assess the presence of bioactive components. All the studied plants contained few and/or all the tested phytochemicals (alkaloids, saponins, tannins, phenolic compounds, phlobatannin, steroids, anthraquinone, terpenoids and cardiac glycosides) as presented in Fig. 1. All the studied phytochemicals were present in either aqueous, methanolic, ethanolic and in all extracts of the plants except anthraquinone that was present in only aqueous extracts of Carica papaya, Afzelia africana and Ageratum conyzoides.

|
These secondary plant metabolites have been reported to contain antibacterial potency and have been actively used or in combination with antibiotics in the therapy of bacterial infections29,30. The demand in the use of natural products as antioxidants and antimicrobial compounds has led to investigate various extracts from many plants, this has recently been of great interest in both research and the food industry, because of growing tendency and possibility to replace synthetic antioxidants and antimicrobials compounds with natural ones31. Many other researchers have reported diverse plants and plants parts solvent extracts containing arrays of these phytochemicals worldwide32,33. The in vitro antioxidants scavenging potentials of H2O2 (%), ABTS (%), nitric oxide (%), DPPH (%), super oxide (%) scavenging activities and concentrations of total phenol and total flavonoids were investigated as reported in Fig. 2. Reactive oxygen species (ROS) is a term that encompasses all highly reactive, oxygen containing molecules, including free radicals like hydroxyl radical, the superoxide anion radical, hydrogen peroxide, singlet oxygen, nitric oxide radical, hypochlorite radical and various lipid peroxides with capability of reacting with membrane lipids, nucleic acids, proteins and enzymes and other small molecules, resulting in cellular damage34. Antioxidant compounds may function as free radical scavengers, initiator of the complexes of prooxidant metals, reducing agents and quenchers of singlet oxygen formation. The in vitro antioxidants scavenging potentials results obtained in this study however corroborated the observations of earlier investigations by other researchers worldwide revealing plants as major sources of antioxidants35-39. Antioxidant’s enzymes (CAT, SOD, GPx, GR and GST) and GSH were also investigated as presented in Fig. 3-8. The results showed the plants extracts exhibited high antioxidant enzyme activities and promising concentration of non-enzyme antioxidant compound (GSH). Antioxidants are molecules that prevent oxidation or inactivates the reactive oxygen species and thus prevent oxidative damage to the cells and body tissues40. It has been reported that catalase (CAT) is an enzymatic antioxidant widely distributed in all animal tissues, decomposes hydrogen peroxide and protects the tissue from highly reactive hydroxyl radicals41.

|
Its activity varies greatly from tissue to tissue, the highest activity is found in liver and kidney, whereas the lowest activity has been reported in the connective tissue42. It was evidenced in this study that the five plants and the three extracts enhanced the activity of CAT in serum as seen in Fig. 3a and all the organs studied in Fig. 3b-d, this is in agreement with what was obtained by Doungue et al.43 and Oseni et al.44. The enhanced catalase activity could be due to the presence of bioactive compounds present in these plants extracts that would give their protons to stabilize radicals formed during stress. Administration of DDVP significantly decrease SOD activity in the studied organs while the five plants solvents extracts caused a concomitant increased activity in the treated groups as presented in Fig. 4a-d. Similar tends were also observed in GPx activity in Fig. 5a-d, GR activity in Fig. 6a-d and GST activity in Fig. 7a-d. The concentration of reduced glutathione (GSH) was also observed to be grossly reduced by the administration of DDVP but treatments with the plants solvents extracts produced upward effects by restoring the GSH concentrations in the serum in Fig. 8a and the studied organs as presented in Fig. 8b-d. In all the antioxidants investigations, the plants solvent extracts reduced the effects of DDVP and hence reversed the oxidative stress expressed by DDVP. From Fig. 9 showing the effects of various extracts of plants on the MDA in DDVP induction, there was increased MDA concentrations in the serum as seen in Fig. 9a and the studied organs presented in Fig. 9b-d to indicate the sign of lipid peroxidation which implicates oxidative stress. On treatments, all the plants extract showed protective role by decreasing the lipid peroxidation level in DDVP induced animals and increased activities of enzymatic (CAT, SOD, GPx, GR and GST) antioxidants and the level of non-enzymatic (GSH) antioxidant. The results obtained in this study however corroborates those reported by other researchers in their various works on different plants extracts and effects on oxidative stress45-49. The results of effects of various extracts of the five plants on the inhibition of acetylcholinesterase activity in the serum and brain of DDVP induced animals was shown in Fig. 10.
|
|
|
The results obtained in this study showed that DDVP induction of 3.3 mg kg–1 b.wt., for 14 days significantly elevated AChE in both serum and brain tissues as seen for all the five studied plants in Fig. 10, it has also been reported by other researchers that DDVP caused increase AChE activity and oxidative stress44,47,49. It has been reported that AChE interact with amyloid β and promotes the formation of amyloid fibril through a pool of amino acids situated in proximity of the peripheral anionic site (PAS) of the enzyme resulting in neurocognitive impairment50. Several studies have revealed that AChE inhibitors not only facilitate transmission of the cholinergic system, but also interfere with the synthesis, aggregation and deposition of toxic amyloid β50. In addition, dysregulation of AChE levels has also been reported in myasthenia gravis, glaucoma, Lewy bodies and Parkinson’s disease51. The observations in this study however corroborated the earlier similar works of Doungue et al.43 in neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract and juice in aluminum chloride-induced Alzheimer’s disease rats, Balamurugan et al.52 in a review on recent updates of acetylcholinesterase inhibitors from plant sources as well as Nwidu et al.53 in anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea.
By implications, the study was undergone to establish the potentials and effectiveness of the five plant species of Ocimum gratissimum, Polyalthia longifolia, Carica papaya, Afzelia africana and Ageratum conyzoides in the treatment of Alzheimer’s and related cognitive complications, the results obtained for the extracts in this study showed that the plants possessed reasonable anti-oxidative and acetylcholinesterase inhibition potentials that could be locally utilized in drug developments for prevention and cure of the consequences of Alzheimer disease (AD) and related cognitive complications. Further purifications could be carried on the plants extracts to isolate their active components using state of the earth equipment.
CONCLUSION
The inhibition of the AChE activity by the plants solvents extracts increased the acetylcholine concentration of the serum and the brain tissues with positive impact on the cognitive function, hence this study clearly showed that these plants solvents extracts possessed AChE inhibition and antioxidant properties thus could reverse DDVP-induced cognitive dysfunction like Alzheimer’s disease and other related cognitive complications.
SIGNIFICANCE STATEMENT
Alzheimer’s Disease (AD) is the most common single cause of dementia in our ageing society, which is traditionally thought of as an untreatable degenerative condition; though recent advances in drug therapy have challenged this view. On the other hand, these drugs may be too expensive and not in the reach of many in sub-Sahara Africa and Nigeria in particular to maintain adequate health conditions. This has called for alternative means of attaining the required global Millennium Developmental Goals (MDGs) status on health and also prevents mortality among the concerned ages. It is therefore needful and unavoidable to investigate and assess naturally sourced and inexpensive edible and functional plants of medicinal importance that can be used to fight and combat the scourge of these problems.
ACKNOWLEDGMENT
Mr. Olayinka Okoh of Department of Chemical Sciences, Faculty of Science and Science Education, Anchor University Lagos is hereby acknowledged for his inputs in results presentations.
REFERENCES
- Dhanasekaran, S., P. Perumal and M. Palayan, 2015. In-vitro screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of Ipomoea aquatica Forsk: Therapeutic lead for Alzheimer's disease. J. Appl. Pharm. Sci., 5: 12-16.
- Murraya, A.P., M.B. Faraonia, M.J. Castroa, N.P. Alzaa and V. Cavallaro 2013. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Cur. Neuropharmcol., 11: 388-413.
- Hua, X., M. Lei, J. Ding, Q. Han, G. Hu and M. Xiao, 2008. Pathological and biochemical alterations of astrocytes in ovariectomized rats injected with D-galactose: A potential contribution to Alzheimer's disease processes. Exp. Neurol., 210: 709-718.
- Volpicelli-Daley, L.A., E.G. Duysen, O. Lockridge and A.I. Levey, 2003. Altered hippocampal muscarinic receptors in acetylcholinesterase-deficient mice. Ann. Neurol., 53: 788-796.
- Heleno, S.A., L. Barros, A. Martins, M.J.R.P. Queiroz, C. Santos-Buelga and I.C.F.R. Ferreira, 2012. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int., 46: 135-140.
- Lahiri, D.K., M.R. Farlow, N.H. Greig and K. Sambamurti, 2002. Current drug targets for Alzheimer's disease treatment. Drug Dev. Res., 56: 267-281.
- Kalauni, S.K., M.I. Choudhary, A. Khalid, M.D. Manandhar, F. Shaheen, Atta-ur-Rahman and M.B. Gewali, 2002. New cholinesterase inhibiting steroidal alkaloids from the leaves of Sarcococca coriacea of Nepalese Origin. Chem. Pharm. Bull., 50: 1423-1426.
- Atta-ur-Rahman, A.T. Wahab, S.A. Nawas and M.I. Choudhary, 2004. New cholinesterase inhibiting bisbenzylisoquinoline alkaloids from Cocculus pendulus. Chem. Pharm. Bull., 52: 802-806.
- Ahmad, W., B. Ahmad, M. Ahmad, Z. Iqbal, M. Nisar and M. Ahmad, 2003. In vitro inhibition of acetylcholinesterase, butyrylxcholinesterase and lipoxygenase by crude extract of Myricaria elegans Royle. J. Biol. Sci., 3: 1046-1049.
- Sung, S.Y., S.Y. Kang, K.Y. Lee, M.J. Park, J.H. Kim and J.H. Park, 2002. (+)-α-Viniferin, a stilbene Trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol. Pharm. Bull., 25: 125-127.
- Okoroiwu, H.U. and I.A. Iwara, 2018. Dichlorvos toxicity: A public health perspective. Interdiscip. Toxicol., 11: 129-137.
- Ekor, M., 2014. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.. 4.
- Sarkar, S., S. Zaidi, A.K. Chaturvedi, R. Srivastava and P.K. Dwivedi et al., 2015. Search for a herbal medicine: Anti-asthmatic activity of methanolic extract of Curcuma longa. Res. Rev.: J. Pharmacogn. Phytochem., 3: 59-72.
- Hussein, R.A. and A.A. El-Anssary, 2019. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In: Herbal Medicine, Builders, P. (Ed.), IntechOpen, London, UK, ISBN: 978-1-78984-783-3.
- Abubakar, R.A. and Mainul Haque, 2020. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. BioAllied Sci., 12: 1-10.
- Evans, W.C., D. Evans and G.E. Trease, 2002. Trease and Evans' Pharmacognosy. 15th Edn., WB Saunders, London, ISBN: 9780702029332, Pages: 585.
- Oseni, O.A., H.A. Emmanuel-Akerele and W.A. Adebayo, 2021. Acute toxicity, phytochemical screening and antimicrobial investigation of aqueous extract of sodom apple tree (Calotropis procera Ait. F) leaf obtained within Anchor University, Lagos. Food ScienTech J., 3: 121-126.
- Nogueira, R.F.P., M.C. Oliveira and W.C. Paterlini, 2005. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate, Talanta, 66: 86-91.
- Shirwaikar, A., A. Shirwaikar, K. Rajendran and I.S.R Punitha, 2006. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull., 29: 1906-1910.
- Jagetia, G.C., S.K. Rao, M.S. Baliga and K.S. Babu, 2004. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: A preliminary study. Phytother. Res., 18: 561-565.
- Oyetayo, F.L., O.A. Oseni, O.S. Akinlolu and D.U. Momodu, 2021. Antidiabetic, antilipidemic and antioxidant properties of aqueous extracts of Morinda lucida and Nauclea latifolia leaves in alloxan induced rats. Biointerface Res. Appl. Chem., 11: 11602-11615.
- Senthilkumar, M., N. Amaresan and A. Sankaranarayanan, 2021. Estimation of Superoxide Dismutase (SOD). In: Plant-Microbe Interactions: Laboratory Techniques, Senthilkumar, M., N. Amaresan and A. Sankaranarayanan (Eds.), Humana, New York, ISBN: 978-1-0716-1080-0, pp: 117-118.
- Madaan, R., G. Bansal, S. Kumar and A. Sharma, 2011. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci., 73: 666-669.
- Iwase, T., A. Tajima, S. Sugimoto, K.I. Okuda and I. Hironaka et al., 2013. A simple assay for measuring catalase activity: A visual approach. Sci. Rep. 3.
- Sedaghatfard, F., S.A. Razavi, M. Hedayati, N. Razmi and A. Rahnama, 2016. Glutathione peroxidase activity assay with colorimetric method and microplate reading format and comparison with chemiluminescence method. Eur. Online J. Nat. Social Sci., 5: 15-21.
- Vontas, J.G., A.A. Enayati, G.J. Small and J. Hemingway, 2000. A simple biochemical assay for glutathione S-transferase activity and its possible field application for screening glutathione S-transferase-based insecticide resistance. Pestic. Biochem. Physiol., 68: 184-192.
- Salbitatani, G., C. Bottone and S. Carfagna, 2017. Determination of reduced and total glutathione content in extremophilic microalga Galdieria phlegrea. Bio-Protocol. 7.
- Garcia, Y.J., A.J. Rodríguez-Malaver and N. Peñaloza, 2005. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods, 144: 127-135.
- Liu, I.X., D.G. Durham and R.M.E. Richards, 2001. Vancomycin resistance reversal in enterococci by flavonoids. J. Pharm. Pharmacol., 53: 129-132.
- Deba, F., T.D. Xuan, M. Yasuda and S. Tawatu, 2008. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control, 19: 346-352.
- Lourenço, S.C., M. Moldão-Martins and V.D. Alves, 2019. Antioxidants of natural plant origins: From sources to food industry applications. Molecules. 24.
- Vaghasiya, Y., R. Dave and S. Chanda, 2011. Phytochemical analysis of some medicinal plants from Western region of India. Res. J. Med. Plant, 5: 567-576.
- Oseni, O.A., 2018. In vitro compositional investigations of antioxidants, phytochemicals, nutritional and minerals in the fruit of Kigelia africana (Lam.) Benth. Int. J. Contemp. Res. Rev., 9: 20259-20268.
- Krishnamurthy, P. and A. Wadhwani, 2012. Antioxidant Enzymes and Human Health. In: Antioxidant Enzyme, El-Missiry, M.A. (Ed.), IntechOpen, London, UK, ISBN: 978-953-51-0789-7.
- Al-Mustafa, A.H. and O.Y. Al-Thunibat, 2008. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci., 11: 351-358.
- Rajurkar, N.S. and S.M. Hande, 2011. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci., 73: 146-151.
- Ashafa, A.O.T., D.S. Grierson and A.K. Afolayan, 2010. In vitro antioxidant activity of extracts from the leaves of Felicia muricata Thunb. an underutilized medicinal plant in the Eastern Cape Province, South Africa. Afr. J. Tradit. Complementary Altern. Med., 7: 296-302.
- Sharma, U.S. and A. Kumar, 2011. In vitro antioxidant activity of Rubus ellipticus fruits. J. Adv. Pharm. Technol. Res., 2: 47-50.
- Aliyu, A.B., J.I. Achika, J.A. Adewuyi, P. Gangas, H. Ibrahim and A.O. Oyewale, 2019. Antioxidants from Nigerian Medicinal Plants: What Are the Evidence? In: Lipid Peroxidation Research, Mansour, M.A. (Ed.), IntechOpen, London, United Kingdom, ISBN: 978-1-83968-548-4, Pages: 128.
- Nandy, S., H.S. Paul, N.R. Barman, B. Chakraborty, 2012. In vitro evaluation of antioxidant activity of Leucas plukenetii (Roth) Spreng. Asian J. Plant Sci. Res., 2: 254-262.
- Naazeri, S., M. Rostamian, B. Yaghmaei and M. Hedayati, 2014. Fluorimetry as a simple and sensitive method for determination of catalase activity. Zahedan J. Res. Med. Sci., 16: 64-67.
- Merghem, M., S. Dahamna and S. Khennouf, 2019. In vivo antioxidant activity of Ruta montana L. extracts. J. Mater. Environ. Sci., 10: 470-477.
- Doungue, H.T., A.P.N. Kengne and D. Kuate, 2018. Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract, and juice in aluminum chloride-induced Alzheimer’s disease rats. Nutrire. 43.
- Oseni, O.A., E.O. Odesanmi, O.I. Oloyede, O.D. Adebayo and M.A.B. Ogundare, 2018. Antioxidant and hepatoprotective activities of Carica papaya (Papaw Leaf) and Loranthus bengwensis (Cocoa Mistletoes) against diclofenac induced hepatotoxicity in rats. Int. J. Life. Sci. Scienti. Res., 4: 1974-1982.
- Oyeyemi, A.O., O.A. Oseni, O.R. Molehin and A.O. Babatunde, 2020. Influence of Polyalthia longifolia (Sonn) leaves on oxidative stress biomarkers in the kidney of cadmium-induced toxicity rats. Comp. Clin. Pathol., 29: 525-532.
- Yadav, P., S.E. Jadhav, V. Kumar, K.K. Kaul, S.C. Pant and S.J.S. Flora, 2012. Protective efficacy of 2-PAMCl, atropine and curcumin against dichlorvos induced toxicity in rats. Interdiscip. Toxicol., 5: 1-8.
- Al-Shiekh, A.A.M., A.A. Al-Shati and M.A.A. Sarhan, 2014. Effect of white tea extract on antioxidant enzyme activities of streptozotocin-induced diabetic rats. Egypt. Acad. J. Biol. Sci. C. Physiol. Mol. Biol., 6: 17-30.
- Tchamgoue, A.D., L.R.Y. Tchokouaha, N. Tsabang, P.A. Tarkang, J.R. Kuiate and G.A. Agbor, 2018. Costus afer protects cardio-, hepato-, and reno-antioxidant status in streptozotocin-intoxicated Wistar rats. BioMed Res. Int. 2018.
- Maysaa, A.H., H.H. Eman, J.K. Naser, Z.A. Dhefaf, H.A. Ali and K.Z. Haider, 2016. Ameliorative effect of Curcuma longa L. rhizomes against biochemical toxicity induced by dichlorvos in female albino rats. J. Chem. Pharm. Sci., 9: 1098-1106.
- Anand, P., B. Singh and N. Singh, 2012. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem., 20: 1175-1180.
- Colovic, M.B., D.Z. Krstic, T.D. Lazarevic-Pasti, A.M. Bondzic and V.M. Vasic, 2013. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol., 11: 315-335.
- Balamurugan, K., B. Sathya and S. Anbazhagan, 2018. A review on recent updates of acetylcholinesterase inhibitors from plant sources. J. Pharm. Sci. Innov., 7: 98-103.
- Nwidu, L.L., E. Elmorsy, J. Thornton, B. Wijamunige, A. Wijesekara, R. Tarbox and W.G. Carter, 2017. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol., 55: 1875-1883.
How to Cite this paper?
APA-7 Style
Oseni,
O.A., Adeleye,
G.S. (2024). Comparative Anti-Oxidative and Acetylcholinesterase Inhibition Potentials of Selected Plants in Treating Alzheimer’s and Degenerative Diseases. Asian Journal of Biological Sciences, 17(1), 53-68. https://doi.org/10.3923/ajbs.2024.53.68
ACS Style
Oseni,
O.A.; Adeleye,
G.S. Comparative Anti-Oxidative and Acetylcholinesterase Inhibition Potentials of Selected Plants in Treating Alzheimer’s and Degenerative Diseases. Asian J. Biol. Sci 2024, 17, 53-68. https://doi.org/10.3923/ajbs.2024.53.68
AMA Style
Oseni
OA, Adeleye
GS. Comparative Anti-Oxidative and Acetylcholinesterase Inhibition Potentials of Selected Plants in Treating Alzheimer’s and Degenerative Diseases. Asian Journal of Biological Sciences. 2024; 17(1): 53-68. https://doi.org/10.3923/ajbs.2024.53.68
Chicago/Turabian Style
Oseni, Olatunde, Abass, and Gbenga Sunday Adeleye.
2024. "Comparative Anti-Oxidative and Acetylcholinesterase Inhibition Potentials of Selected Plants in Treating Alzheimer’s and Degenerative Diseases" Asian Journal of Biological Sciences 17, no. 1: 53-68. https://doi.org/10.3923/ajbs.2024.53.68

This work is licensed under a Creative Commons Attribution 4.0 International License.



