Biomarkers of Liver Status in Heavy Alcoholics Drinkers Resident in Bayelsa State, Nigeria
| Received 29 Dec, 2023 |
Accepted 21 Feb, 2024 |
Published 31 Mar, 2024 |
Background and Objective: The wide variety of biomarkers reflecting liver status is known to be influenced by heavy alcohol consumers and the dose-response relationships between alcohol intake and marker changes have not been fully elucidated. Therefore, the study aimed to evaluate the Biomarkers of liver status in heavy alcoholic drinkers resident in Bayelsa State. This will help us develop a diagnostic tool for alcohol-related disorders with positive management outcomes that will aid us in forming a diagnostic tool for the diseases associated with alcohol consumption with good management outcomes. Materials and Methods: The study included 200 male and female subjects ranging in age from 15 to 65 years. A well-structured pre-tested questionnaire was used to collect information that was used to categorise participants into four groups: Non-alcohol consumers (one drink per month), occasional alcohol consumers (1-3 drinks per month), moderate alcohol consumers (1-5 drinks per week) and heavy alcohol consumers (>2 drinks per day). Results: Aspartate Aminotransferase (AST) (11.54±3.63 U/L) and Alanine Aminotransferase (ALT) (6.30±1.79 U/L) were significantly higher in group 4 and progressively from group 2 to 4 when compared to control (aspartate aminotransferase (5.28±1.28 U/L), alanine aminotransferase (2.86±0.76 U/L). The AST and ALT were all significantly higher in the test groups when compared to control. Conclusion: The study concluded that chronic alcohol consumption alters aspartate aminotransferase and alanine aminotransferase (liver enzymes) affecting the function of the liver and hence can be used as a biomarker for heavy alcohol drinkers.
| Copyright © 2024 Maduka and Onuoha. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Alcohol, occasionally denoted by the chemical name ethanol, is a psychoactive drug that is the active ingredient in drinks such as beer, wine and distilled spirits (hard liquor)1. It is considered one of the ancient and most common recreational substances, causing the distinctive effects of alcohol intoxication ("drunkenness")2.
Research has shown that there is an overwhelming link between excessive use of alcohol and a wide range of harmful outcomes such as alcohol use disorder, alcoholic liver disease, vehicular accidents, a host of social and legal problems, mortality and morbidity from chronic medical conditions3.
With various submissions, the liver as an organ is considered to be the earliest and the greatest predisposed to varying degrees of tissue injury from excessive alcohol drinking because of its unique function as a primary site of ethanol metabolism4. These adverse effects of alcohol on the liver by various authors have been considered as either long-term or short-term; short-term adverse effect includes dizziness, nausea and vomiting and can result in independence and withdrawal while long-term adverse effect includes brain damage5, liver damage6 and increased risk of cancer7.
Alcoholic liver disease (ALD) a broad term used to describe alcohol-related liver pathology has been deliberated as the cause of increased morbidity and mortality and accounts for elevated social and economic costs3. The ALD has a wide clinical and histopathological spectrum. Fatty liver (steatosis), which is reversible with abstinence, is at one end of the range, while alcoholic hepatitis and fibrosis, which may or may not improve with abstinence, are at the other. Cirrhosis (Laennec’s cirrhosis) and end-stage liver disease, on the other hand, are illnesses that are usually irreversible and have a bad prognosis. Furthermore, consuming alcohol while being obese increases the risk of getting many types of cancer, particularly hepatocellular carcinoma3.
Therefore, the study aimed to evaluate the biomarkers of liver status in heavy alcoholic drinker’s residents in Bayelsa State that will aid us informing a diagnostic tool for the diseases associated with alcohol consumption with good management outcome.
MATERIALS AND METHODS
Study area: This study was carried out in communities of Bayelsa State coordinate 4.8678°N, 5.8987°E. Bayelsa State is a state in Southern Nigeria in the core Niger Delta Region between Delta State and River State with a total land mass of about 10,773 km2 (4,159 sq mi). Its capital is Yenagoa on coordinates 4°55'29N, 6°15'51E. The main language spoken is Ijaw language having English as the official language. The state was created in 1996 from part of Rivers State with an estimated population of 1,704,515 according to the 2006 census8. Bayelsa State has eight Local Government Areas and the state’s capital is Yenagoa. The study was conducted from April 2018 to May 2021.
Study population: The study population comprised males and females within the age bracket of 15-65 years who consume alcohol and alcohol-based products and a control group comprised of individuals who have not consumed alcohol or alcohol-based products. The study populations of two hundred participants were divided into 4 group:
Group 1 |
: |
Fifty non-alcohol consumers (<1 drink monthly) |
|
Group 2 |
: |
Fifty occasional alcohol consumers (1-3 drinks/month) |
|
Group 3 |
: |
Fifty moderate alcohol consumers (1-5 drinks/week) |
|
Group 4 |
: |
Fifty heavy alcohol consumers (>2 drinks/day) |
Inclusion criteria:
• |
All consenting individuals between the ages of 15 and 65 who use alcohol with no physical signs of sickness |
|
• |
All consenting adults who do not consume alcohol and are of legal drinking age |
Exclusion criteria:
• |
Individuals with reported or confirmed cases of liver illness, myocardial infarction, inflammatory bowel disease, immunological deficiencies or on antiretroviral medication and tuberculosis |
|
• |
Pregnant women or women using contraception |
|
• |
Individuals taking anticoagulant therapy, cytotoxic drugs, anti-diabetic therapy and anti-hypertensive therapy were excluded from the trial, as were non-consenting individuals |
Sample size: The following formula, provided by May and Looney9 was employed:
Where, Z is the critical value and in a tailed least, this is equivalent to 1.95, P is estimated prevalence of alcoholics in the Niger Delta Region (3.2%), q represents the probability, which is 1-p, d is the absolute sampling error that can be accepted. It will be set at 5% more than the minimum sampling size N in this study.
 |
A minimum sample size of 48 is required, but for this study, 50 participants in each group were recruited at random in localities throughout Bayelsa State. The test group consists of people who consume alcohol and alcohol-based goods, while the control group does not consume alcohol or alcohol-based products.
Collection of sample: Aseptically, using 5 mL syringe, 3 mL of venous blood was collected, dispensed into a lithium heparin container sample for liver function test and was centrifuged at 4000 rpm for 15 min, plasma was separated and frozen at -20°C until when required for analysis.
Ethical approval: With a letter of introduction from the Head of Medical Laboratory Science Department, University of Benin ethical clearance was obtained from the Bayelsa State Ministry of Health and ethical research committee for the approval to collect samples from consenting individuals at communities in Bayelsa State.
Method of analysis
Alanine Aminotransferase (ALT)
Principle: The ALT in serum is incubated at 37°C for exactly 30 min in a pH 7.4 phosphate-buffered substrate containing alanine and α-oxoglutarate. The ALT catalyzes the transfer of the amino group from alanine to oxoglutarate, forming pyruvate and glutamate10:
The pyruvate reacts with 2, 4-Dinitrophenylhydrazine (DNPH) to form pyruvate hydrazone. The ALT is measured by monitoring the concentration of pyruvate hydrazone formed with DNPH which in an alkaline medium gives a red-brown colour. The absorbance of the colour produced is measured using a spectrophotometer (Infitek Company, Jinan, Shandong, China) at a wavelength of 546 nm.
Aspartate Amino Transferase (AST)
Principle: The AST in serum is incubated at 37°C for exactly 30 min in a pH 7.4 buffered substrate containing aspartate and α-oxoglutarate. The AST catalyzes the transfer of amino groups from aspartate to oxoglutarate, forming oxaloacetate and glutamate10:
The oxaloacetate reacts with 2, 4-Dinitrophenylhydrazine (DNPH) to form oxaloacetate hydrazone which the colour produced is measured using a spectrophotometer at a wavelength of 546 nm.
Statistical analysis: Statistical package for Social Sciences (SPSS) (Version 20.1 for Windows 10) was used to analyze data, differences in the various parameters were evaluated using Kolmogorov-Simirnov Z statistics, One-way ANOVA was used to assess differences within the group and statistically significant values were determined at 95% confidence level.
RESULTS
Table 1 shows the mean and standard deviation (SD) of some liver function test enzymes of participants. Highest mean and SD of aspartate aminotransferase (11.54±3.63 U/L) and alanine aminotransferase (6.30±1.79 U/L) were obtained in group 4 (heavy alcohol consumers) while least mean and SD aspartate aminotransferase (5.28±1.28 U/L) and alanine aminotransferase (2.86±0.76 U/L) was obtained in control group (non-alcohol consumers). Analysis of Variance (ANOVA) showed a statistically significant difference (p<0.05) in comparison of the control mean and SD values of aspartate aminotransferase and alanine aminotransferase to other participant groups.
Table 2 shows the post-hoc (Turkey’s LSD Test) comparison of the effect of alcohol consumption on some liver function test enzymes among participants. It was compared at a statistical confidence level of 95%. The AST and ALT were all significantly increased when compared to control (non-alcohol consumers). Interanalysis indicated statistically significant differences for all inter comparisons.
| Table 1: | Mean and SD of some liver function test enzymes of participants | |||
| Group (n = 200) | AST (U/L) |
ALT (U/L) |
| Group 1 | 5.28±1.28 |
2.86±0.76 |
| Group 2 | 9.10±2.36 |
4.28±0.95 |
| Group 3 | 10.32±2.30 |
5.29±1.07 |
| Group 4 | 11.54±3.63 |
6.30±1.79 |
| p-value | 0 |
0 |
| F-value | 57.32 |
74.239 |
| All values were expressed as Mean±SD for participants across all the groups, *Statistically significant difference between means as compared with control (p<0.05), Group 1 (Control): Non-alcohol consumers (<1 drink monthly), Group 2: Occasional alcohol consumers (1-3 drinks/month), Group 3: Moderate alcohol consumers (1-5 drinks/week) and Group 4: Heavy alcohol consumers (>2 drinks/day) | ||
| Table 2: | post-hoc (Turkey’s LSD Test) comparison of effect of alcohol consumption on some liver function test enzymes among participants | |||
| Group | AST (U/L) |
ALT (U/L) |
| Group 1 vs 2 | 0.000a |
0.000a |
| Group 1 vs 3 | 0.000a |
0.001a |
| Group 1 vs 4 | 0.000a |
0.000a |
| Group 2 vs 3 | 0.017a |
0.000a |
| Group 2 vs 4 | 0.001a |
0.000a |
| Group 3 vs 4 | 0.017a |
0.001a |
| aMean difference is significant at the 0.05 level, Group 1 (Control): Non-alcohol consumers (<1 drink monthly), Group 2: Occasional alcohol consumers (1-3 drinks/month), Group 3: Moderate alcohol consumers (1-5 drinks/week) and Group 4: Heavy alcohol consumers (>2 drinks/day) | ||
|

|
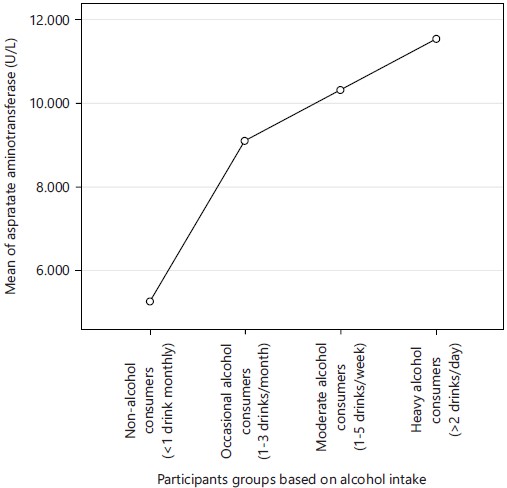
Figure 1 shows mean plot graph of participants groups against mean aspartate aminotransferase value (U/L). Highest mean of aspartate aminotransferase (11.54 U/L) was obtained in group 4 (heavy alcohol consumers) while least mean aspartate aminotransferase (5.28 U/L) was obtained in control group (non-alcohol consumers).
Figure 2 shows mean plot graph of participants groups against mean alanine aminotransferase value (U/L). Highest mean of alanine aminotransferase (6.30 U/L) were obtained in group 4 (heavy alcohol consumers) while least mean alanine aminotransferase (2.86 U/L) was obtained in control group (non-alcohol consumers).
DISCUSSION
This present study indicates that heavy alcohol consumers show higher enzyme activities than moderate alcohol consumers, occasional alcohol consumers and non-alcohol consumers as seen in Fig. 1 and 2. Furthermore, moderate alcohol consumers also show higher enzyme activities than occasional alcohol consumers and non-alcohol consumers underscoring an early occurrence of the biochemical reactions in response to alcohol intake.
The observation of this study shows changes in liver function test enzyme aspartate transaminase and alanine transaminase. Tissue damage is typically coupled with the release of enzymes unique to the afflicted tissue or organ into the circulatory system. As a result, the activity of such enzymes in body fluids increases11. Liver cell damage manifests as a decrease in the permeability of Aspartate and Alanine Aminotransferase (AST and ALT, respectively), which are liver marker enzymes. Tissue damage is frequently accompanied by the release of enzymes unique to the afflicted tissue or organ in circulation. The result is an increase in the activity of such enzymes in bodily fluids11. Liver cell death manifests itself as a decrease in the permeability of Aspartate and Alanine Aminotransferases (AST and ALT, respectively), which are liver marker enzymes. Measurement of enzymatic activities of aminotransferase (AST and ALT) are of clinical and toxicological importance, as changes in their activities are indicative of tissue damage by toxicants or in disease conditions12. The AST and ALT were all significantly increased in all participant groups when compared to control (non-alcohol consumers). These findings were in agreement with various authors who suggested the increased activity of these enzymes to the unique role of a hepatic cell as the primary site of alcohol metabolism12-14. Most of the current studies were still in agreement with studies indicating that aspartate and alanine aminotransferase (AST and ALT) as biomarkers for disorders associated with heavy alcohol drinkers15-19. This implies that Alcohol consumption may induce hepatic disorders such as necrosis or inflammation as liver function test enzymes aspartate transaminase and alanine transaminase were significantly increased.
Therefore, it can be applied as a biomarker for heavy alcohol drinkers which can be of diagnostic assistance in the management of alcohol associated diseases. It is recommended that there should be a public campaign against alcohol consumption starting from primary school to tertiary institutions, in the market places, motor parks and other public places. Lack of funds limits the study only to two liver enzymes, more studies can be carried out on other enzymes.
CONCLUSION
The study concluded that chronic alcohol consumption changes liver enzymes including aspartate aminotransferase and alanine aminotransferase affecting the function of the liver and hence can be used as a biomarker for heavy alcohol drinkers. It is recommended that more research be carried out for other liver enzymes to establish their diagnostic tool.
SIGNIFICANCE STATEMENT
Alcohol consumption and associated medical disorders continue to grow in Nigeria, most especially Niger Delta Region. The effects of alcohol consumption on liver enzymes discovered that aspartate aminotransferase and alanine aminotransferase as biomarkers in liver status in heavy alcoholic drinkers. This can be of diagnostic assistance in the management of alcohol associated diseases. This study will help the researcher uncover the critical area of consumption of alcohol severity that many researchers were not able to explore. Thus, a new theory on effect of consumption on liver enzymes may be arrived at.
REFERENCES
- Collins, S.E. and M. Kirouac, 2013. Alcohol Consumption. In: Encyclopedia of Behavioral Medicine, Gellman, M.D. and J.R. Turner (Eds.), Springer, New York, ISBN: 978-1-4419-1005-9, pp: 61-65.
- Ajayi, A.I., E.O. Owolabi and O.O. Olajire, 2019. Alcohol use among Nigerian university students: Prevalence, correlates and frequency of use. BMC Public Health, 19.
- Keyes, K.M., E. Calvo, K.A. Ornstein, C. Rutherford, M.P. Fox, U.M. Staudinger and L.P. Fried, 2019. Alcohol consumption in later life and mortality in the United States: Results from 9 waves of the health and retirement study. Alcohol. Clin. Exp. Res., 43: 1734-1746.
- Osna, N.A., T.M. Donohue Jr. and K.K. Kharbanda, 2017. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res., 38: 147-161.
- Singal, A.K. and S.M. Bailey, 2018. Cellular abnormalities and emerging biomarkers in alcohol-associated liver disease. Gene Express., 19: 49-60.
- Louvet, A. and P. Mathurin, 2015. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol., 12: 231-242.
- Rachdaoui, N. and D.K. Sarkar, 2017. Pathophysiology of the effects of alcohol abuse on the endocrine system. Alcohol Res.: Curr. Rev., 38.
- Akintujoye, E.O., 2012. Perception and utilization of traditional birth attendants by pregnant women attending primary health care clinics in a rural Local Government Area in Ogun State, Nigeria. Int. J. Women's Health, 4: 25-34.
- May, J.O. and S.W. Looney, 2022. On sample size determination when comparing two independent spearman or Kendall coefficients. Open J. Stat., 12: 291-302.
- Reitman, S. and S. Frankel, 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol., 28: 56-63.
- Aliyu, R., A.H. Adebayo, D. Gatsing and I.H. Garba, 2007. The effects of ethanolic leaf extract of Commiphora africana (Burseraceae) on rat liver and kidney functions. J. Pharmacol. Toxicol., 2: 373-379.
- Salehi, B., A. Ata, N.V.A. Kumar, F. Sharopov and K. Ramírez-Alarcón et al., 2019. Antidiabetic potential of medicinal plants and their active components. Biomolecules, 9.
- Agarwal, S., V.L. Fulgoni III and H.R. Lieberman, 2015. Assessing alcohol intake & its dose-dependent effects on liver enzymes by 24-h recall and questionnaire using NHANES 2001-2010 data. Nutr. J., 15.
- Gogoi, J.B., M. Bhasin, J.K. Behera, K. Gairola and H. Baruah, 2018. A study of effect of alcohol on liver function tests (LFT) in Garhwal Hills, India. Int. J. Res. Med. Sci., 6: 94-98.
- Kherada, S., S. Sharma, S. Gocher and L.C. Bairwa, 2020. Correlation of type, quantity, and duration of alcohol consumption with biochemical markers and liver function tests. Prim. Care Companion CNS Disord., 22.
- Rosoff, D.B., K. Charlet, J. Jung, J. Lee and C. Muench et al., 2019. Association of high-intensity binge drinking with lipid and liver function enzyme levels. JAMA Netw. Open, 2.
- Sharma, P. and A. Arora, 2020. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: Spectrum and diagnosis. Transl. Gastroenterol. Hepatol., 5.
- Elbendary, E.Y., M.H. Mahmoud, S.F. Salem and A.M. Farah, 2023. The effects of energy drink consumption on kidney and liver function: A comparative study. J. Biosci. Med., 11: 171-181.
- Ganesh, S., N. Joshi, M.K. Jain, L. Sharma and A. Desai et al., 2022. Clinical and safety evaluation of Liv.52 in alcoholic liver disease: A review. Gastroenterol. Insights, 13: 377-386.
How to Cite this paper?
APA-7 Style
Maduka,
V.A., Onuoha,
E.C. (2024). Biomarkers of Liver Status in Heavy Alcoholics Drinkers Resident in Bayelsa State, Nigeria. Asian Journal of Biological Sciences, 17(1), 138-144. https://doi.org/10.3923/ajbs.2024.138.144
ACS Style
Maduka,
V.A.; Onuoha,
E.C. Biomarkers of Liver Status in Heavy Alcoholics Drinkers Resident in Bayelsa State, Nigeria. Asian J. Biol. Sci 2024, 17, 138-144. https://doi.org/10.3923/ajbs.2024.138.144
AMA Style
Maduka
VA, Onuoha
EC. Biomarkers of Liver Status in Heavy Alcoholics Drinkers Resident in Bayelsa State, Nigeria. Asian Journal of Biological Sciences. 2024; 17(1): 138-144. https://doi.org/10.3923/ajbs.2024.138.144
Chicago/Turabian Style
Maduka, Vivian, Akudo, and Emmanuel Chinedu Onuoha.
2024. "Biomarkers of Liver Status in Heavy Alcoholics Drinkers Resident in Bayelsa State, Nigeria" Asian Journal of Biological Sciences 17, no. 1: 138-144. https://doi.org/10.3923/ajbs.2024.138.144

This work is licensed under a Creative Commons Attribution 4.0 International License.



