Impacts of Photoperiod Manipulation on Growth and Welfare of Juvenile African Catfish
| Received 15 Mar, 2024 |
Accepted 15 May, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Photoperiod can influence the growth and welfare of Clarias gariepinus. This study aims to investigate the response of African catfish to different photoperiodic regimes with regard to growth performance. Materials and Methods: Clarias gariepinus was reared in triplicate under three photoperiods: 24 hrs total light (24L:0D); 12 hrs of light and 12 hrs of darkness (12L:12D) and 24 hrs of total darkness (24D:0L) fed at 5% body weight. The experiment lasted for 18 weeks. Statistically, data was analyzed using Analysis of Variance and Duncan’s Multiple Range Test was used to separate means. Results: From the statistical analysis, significant differences (p<0.05) were observed in some parameters while others were not significantly different. The 12D:12L photoperiod group showed the highest values for growth, net production value (NPV), gross profit (GP), profit index (PI) and benefit cost ratio (BCR), while those under 24L:0D showed the least values. Levels of cortisol were highest and least in 0D:24L and 12D:12L, respectively. As the number of hours of light exposure increased, glucose level increased. This is shown in 00D:24L having the highest glucose level. Conclusion: The study revealed that the simple, low-cost technique of a 12D:12L photoperiod could be applied by farmers to achieve faster growth in less time in view of maximizing profit.
| Copyright © 2024 Afia et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Considering the economic importance, growth and scale of the aquaculture industry particularly, it is necessary to properly evaluate the effect of rhythmicity and alternation of photoperiod regimes on fish health and welfare1. The concept of fish welfare is complex and hinges on an individual animal’s perception of its quality of life. This can vary from the degree of being very good to very poor depending on the environmental challenges it faces2. Kendra and Josh3 refer to welfare as the status of a good life for animals. This quality cuts across several aspects of life, such as health, happiness and longevity. According to Bano and Serajuddin4, fish welfare comprises the fish’s health, stability, adequate nourishment, safety and ability to exhibit natural tendencies, while avoiding negative states that would inflict pain and discomfort. In recent years the importance of welfare in fish aquaculture has increased dramatically and most intensive farming operations operate under welfare standards5.
Fish growth is influenced by several biotic and abiotic factors6. The physical factor such as photoperiod is known to increase the growth and survival rate of juvenile fish, particularly in temperate regions through processes such as photostimulation and feed intake which improves the feed conversion efficiency7. For some species, long photoperiod regimes might indirectly modify growth by eliciting an increased feed intake, developing muscle mass through increased locomotor activity8, enhancing nutrient use efficiency9 and/or redirecting energy from gonadal development into somatic growth6.
Aquatic organisms’ growth and survival can also be impacted by environmental factors. Different levels of light intensity can offer significant benefits to catfish10. The photoperiod, being an external factor, can directly impact fish growth by altering hormone secretion and endocrine function9,11. Photoperiod regulates growth by affecting internal rhythms and the levels of growth hormone in circulation12. The photoperiod refers to the daily alteration of light and darkness and significantly influences the hatchability of eggs, as well as the growth and survival of fish13. The variation between light and darkness is a crucial factor that governs numerous biological processes in fish14. According to Puvanendran and Brown15 photoperiod has been found to exert a significant effect on the survival of fish larvae and also exert a definite influence on fish metabolism, maturation, behaviour and even coloration. Recent studies have shown that continuous photoperiod is extensively used in fish farming, to regulate the reproductive cycle16. Following Simensen et al.12 report, photoperiod acts as a guiding factor, controlling growth as a zeitgeber (cue or synchronizer), by affecting internal rhythms and the circulating levels of growth hormone and Hisar et al.17 linked the production of melatonin to periods of illumination.
Photoperiod requirements by fish are species-specific and thus vary for each developmental stage. Light and dark alternation is generally thought to be the main synchronizer of feeding activity18. Photoperiod does not only affect the feeding pattern of fish, but also plays a decisive role in growth, survival and social behaviour19. Such influences can be caused by physiological mechanisms; such as altered hormone production, which may improve feed conversion efficiency20. In addition, application of artificial light induces stress that eventually leads to immunosuppression and leaves salmonids and Nile tilapia prone to diseases16,21. However, other studies in rainbow trout Oncorhynchus mykiss under a constant light regime show an increase in plasma cortisol concentration22 other studies in trout and red sea bream (Pagrus major) show unchanged cortisol levels with no evidence of a stress response23.
Catfish have different luminous preferences and this directly influences their growth, survival, skin pigmentation, behavior and seed quality24,25. Previous studies performed on the effect of photoperiod on growth performance of C. gariepinus concluded that growth can be affected by day length with fish performing better under short-day photoperiods26. Appelbaum and Kamler27 report indicated that C. gariepinus raised in darkness grew larger compared to those raised in the light whereas Almazan-Rueda et al.28 demonstrated that the absence of light led to increased growth of this species Davie et al.29 also noted significant growth rates of C. gariepinus fingerlings when cultured continuously in darkness.
Clarias gariepinus is one of the most commonly cultured, indigenous species of fish in Africa. This species is selected for its resilience, omnivorous diet, capability to consume various natural and man-made feeds, toughness against disease outbreaks and tolerance to low oxygen and pH levels30. Photoperiod adjustment is one of the simple, low-cost techniques that can be used to obtain faster growth during the intense production of C. gariepinus fingerlings. Adebayo31, portrays photoperiodism as one of the physical factors which act in favour of the growth and survival of fish larvae. The body pigmentation and gonadal activity in most species are equally affected by photoperiodism32. Very dark body coloration of fish species such as C. gariepinus coupled with high body mass results in better market value and higher prices.
This study, therefore, aims to extend the bounds of knowledge on the effects of varying photoperiod regimes on juveniles of C. gariepinus in terms of growth and welfare and how these can be harnessed in aquaculture for improved fish production.
MATERIALS AND METHODS
Study area: The experiment was carried out in the Department of Fisheries and Aquaculture Research Farm University of Uyo, Uyo Metropolis, Akwa Ibom State, Nigeria and lasted for 18 weeks (November 2021 to February 2022).
Experimental fish: A total number of one hundred and eighty fingerlings of Clarias gariepinus with an average weight of 1.12 g were used. Coppens was the only commercial feed used throughout the 18 weeks of the feeding trial. The fishes were fed different pellet sizes with respect to their sizes ranging from 0.8-4 mm.
Experimental procedures and design: The fish groups were allocated to nine rearing tanks linked to the water circulation system. The nine tanks were assigned to three photoperiods namely 24 hrs of light (00D: 24L), twelve hours of light 12 hrs of darkness (12D: 12L) and 24 hrs of darkness (24D: 00L). The light phase utilized a (60 W) energy bulb emitting a light intensity of 150 lux measured at the water’s surface, while the dark phase involved completely covering the designated rearing house with tarpaulin material to reduce light intensity to 5 lux at the water’s surface.
Growth performance parameters
Survival rate: The survival rate and other parameters of the fish were determined at the end of the experiment according to Ofor and Afia30 as follows:
 |
Where:
| Nt1 | = | Number of fingerlings stocked | |
| Nt2 | = | Number of fingerlings remaining |
The fish weight gain (WG) was calculated as the difference between the final weight of the fish at the end of the experiment and the initial weight gain in grams thus:
Specific growth rate (SGR): The specific growth rate (SGR) was calculated using the formula in Ofor and Afia30:
Where:
| W1 | = | Initial weight | |
| W2 | = | Final weight | |
| T1-T2 | = | Number of days |
(duration of the experimental period in days)–1
Daily growth rate (DGR): Daily growth rate was calculated using the formula in Ofor and Afia30:
Where:
| FW | = | Final weight | |
| IW | = | Initial weight | |
| EP | = | Experimental period |
 |
Feed utilization indices:
 |
Where:
| Wi | = | Initial mean weight | |
| Wf | = | Final mean weight | |
| t | = | Period of rearing |
Rearing period in days
Where:| SR | = | Survival rate | |
| MFW | = | Mean final weight | |
| MIW | = | Mean initial weight |
 |
Economic indices: A simple economic analysis was developed to estimate the profitability in each treatment using the formula in Sebastian et al.35:
Net production value (NPV): This was taken as the cost of all the fish harvested at the end of the experiment as follows:
Gross profit (GP): This was taken as the difference between the net profit value and investment cost analysis in terms of the projected cost of the fish raised:
Statistical analysis: Data obtained from this experiment were subjected to a one way Analysis of Variance (ANOVA). Duncan’s Multiple Range Test (DMRT) was used to determine the difference between the means (p<0.05) where significant.
RESULTS
Growth performance and nutrient utilization parameters of C. gariepinus under three photoperiod regimes: The parameters for growth and nutrients utilization, IMW, FMW, MWG, RGR, RWG, FCR, SR, SGR and PER of catfish C. gariepinus were not significantly different (p>0.05) for the different phototropic regimes (Table 1) except for metabolic growth rate.
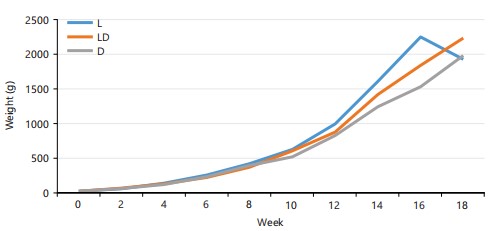
The IMW, WFW and MWG values were all highest in 12D:12L photoperiod regime while the lowest values were recorded in 24D: L for IMW, 0D:24L for FMW and MWG respectively (Fig. 1).
Mean weight gain: The growth response of Clarias gariepinus subjected to three photoperiods is presented in Table 1. The mean weight gain varied from 164.46±9.72 to 174.80±1.43 to 167.63±8.02 for L, LD and D, respectively, with no significant (p>0.05) difference in the mean weight gain across the three photoperiod regimes. The highest mean weight gain was recorded in 12L: 12D with a value of 174.80±1.43 and the lowest 164.46±9.72 in 12L:0D photoperiod regime.
Food conversion ratio: The highest feed conversion ratio of 3.06±0.180 was recorded in 0D:24L. and the lowest (2.457±0.04) was obtained in 12D:12L. The result showed that 12D:12L photoperiod had the best feed conversion ability with the lowest value of FCR. There was no significant (p>0.05) difference between the feed conversion ratio in the three photoperiod regimes.
Specific growth rate: The results of the specific growth rate of the experimental fish are presented in Table 1. The highest SGR value 5.47±0.02 was recorded in 12D:12L photoperiod, while the lowest was in 0D: 24L photoperiod with 5.28±0.06. There was no significant (p>0.05) difference between the different photoperiod regimes in the experimental fish.
Protein efficiency ratio: The highest protein levels were observed in 12D: 12L (Table 1) and lowest 0.56±0.03 in 0D:24L group with no significant difference at p>0.05 between the various photoperiod regimes.
Survival rate: The highest level of survival was recorded in 12D:12L (63.33±1.66) while photoperiod regime of 0D:24L and 24D:0L had the least survival rate (Table 1).
|
| Table 1: | Growth performance and nutrient utilization of C. gariepinus under different photoperiod regimes | |||
| Parameter | L | LD | D |
| IW | 1.12±0.01a | 1.12±0.01a | 1.12±0.01a |
| FW | 165.58±9.72a | 175.93±1.43a | 168.76±8.02a |
| MWG | 164.46±9.72a | 174.80±1.43a | 167.63±8.02a |
| Relative growth rate | 8409.21±498.47a | 9814.79±175.72a | 8670.25±912.78a |
| Metabolic growth rate | 4.13±3.03b | 4.29±1.21a | 5.23±0.84a |
| SGR | 5.28±0.06a | 5.47±0.02a | 5.31±0.13a |
| RWG | 616.53±169.12a | 530.76±52.79a | 445.46±117.05a |
| Survival rate | 58.33±4.40a | 63.33±1.67a | 58.33±4.41a |
| FCR | 3.06±0.18a | 2.45±0.05a | 2.53±0.24a |
| PER | 0.56±0.03a | 0.70±0.01a | 0.69±0.06a |
| Means with different superscripts along the same row are significantly different (p<0.05), SGR: Specific growth rate, RWG: Relative weight gain, FCR: Feed conversion ratio, PER: Protein efficiency ratio, IW: Initial weight, WF: Final weight and MWG: Mean weight gain | |||
| Table 2: | Economic production indices of C. gariepinus exposure to three different photoperiod regimes | |||
| Parameter | L | LD | D |
| Cost of feed | 7243.36±179.63a | 6797.52±244.23a | 6082.09±125.86b |
| ECR | 22184.70±1348.83a | 16724.27±918.84b | 15353.86±1176.74b |
| ICA | 7843.36±179.63a | 7397.52±244.23a | 6682.09±125.86b |
| NPV | 2468830.00±161879.02a | 2866673.33±56062.28a | 2539030.00±264994.76a |
| GP | 2460986.63±161783.75a | 2859275.80±55838.83a | 2532347.90±264881.55a |
| PI | 340.69±19.06a | 422.25±8.07a | 416.16±36.78a |
| BCR | 314.61±17.70a | 387.89±6.42a | 378.88±34.02a |
| *Means with different superscripts along the same row are significantly different (p<0.05), ICA: Investment cost analysis, ECR: Economic conversion ratio, NPV: Production value, GP: Gross profit, PI: Profit index and BCR: Benefit cost ratio | |||
Relative mean rate: There were no significance difference (p>0.05) across the different photoperiod regimes. However, the highest value was recorded in 12D:12L (9814.79±175.72), while the least value (8409.20±498.47) was recorded in 0D:24L.
Metabolic growth rate: The highest MGR of 5.226±0.84312 was recorded in 24D:0L while the lowest (-4.13±3.03) was obtained in 0D:24L and was significantly difference at p<0.05 between the various photoperiod regimes.
Relative weight gain: From the result in Table 1 and Fig. 1, the highest RGW of (616.53±169.12) was recorded in 0D:24L while the lowest (445.46±117.05) was obtained in 24D:0L photoperiod regime. However, there was no significant difference in the means (p>0.05) on RGW across the different photoperiods in the experimental fish.
| Table 3: | Stress indicators of the experimental fish under the three photo period regimes | |||
| Parameter | L | LD | D |
| FBS (glucose) | 4.03±0.49a | 3.16±0.53a | 3.70±0.66a |
| Protein (total) | 35.66±1.20a | 30.33±0.88b | 38.00±1.52a |
| Cortisol (μg/dL) | 15 | 12.7 | 13.5 |
| *Means with different superscripts along the same row are significantly different (p<0.05) | |||
Economic performance of C. gariepinus under different photoperiod regimes: The result of the aspect of economic indices of C. gariepinus fed at 5% body weight exposed to three different photoperiod regimes is presented in Table 2. The result showed significant differences (p<0.05) in the economic conversion ratio (ECR), ICA and cost of feed. However, there were no significant differences (p>0.05) in net production value (NPV), gross profit (GP), profit index (PI) and benefit cost ratio (BC). The maximum cost of feeding (7243.36±179.63), ECR (22184.70±1348.83) and ICA (7843.36±179.63) were recorded in the 0D:24L photoperiod groups, respectively. However, the highest PI (422.25±8.07), GP (2859275.80±55838.83), NPV (2866673.33±56062.28) and BCR (387.89±6.42) were observed in the 12D:12L group, while the highest ICA (7843.36±179.63) was recorded in the 0D:24L group.
From Table 3, there was no significant difference (p>0.05) in FBS (Glucose) across the three photoperiod regimes in this study with the highest value (4.03±0.49) and lowest value (3.16±0.53) observed in (0D:24L) and (12D:12L) of the photoperiod regimes, respectively. Cortisol was highest (15.0 ug/dL) in 0D:24L and lowest (12.7 ug/dL) in 12D:12L photoperiod regimes. There was a significant difference in total protein (p<0.05) across the different photoperiod regimes. The maximum protein (38.00±1.52) was recorded in 24D:0L, while the 12D:12L had the least protein of 30.33±0.88.
DISCUSSION
The findings of the present study showed that the parameters for growth and nutrient utilization: FCR, SR, PER, SGR, IMW, FMW, MWG, RGR and RWG of C. gariepinus juvenile were not influenced (p>0.05) by the different photoperiod regimes except for metabolic growth rate. Photoperiod effects on growth in fish are clearly species-specific and are contingent on the daily activity rhythms (diurnal/nocturnal) and probably the light sensitivity which has been shown to differ greatly between species. The influence of photoperiod on fish growth varies significantly between species, depending on their daily activity patterns and likely differences in light sensitivity, as demonstrated by Migaud et al.36. Fish exposed to 12D:12L had the highest maximum weight gain (2205.13±43.12) at the end of the experiment compared to 24D:0L (1953.10±203.84) and 0D:24L (1899.10±124.52) photoperiod regimes (Table 1). There were striking differences in the initial and final growth of the fish under experiment when they were assessed based on different photoperiod regimes. Similar results were recorded in the case of MGR weight gain (WG) which suggested that the C. gariepinus required several weeks to acclimatize to new rearing conditions. Similar results were reported by Kashyap et al.37 for Catla catla, Bano and Serajuddin4 for Giant Gourami, Trichogaster fasciata.
The FCR (2.45±0.04) value was recorded in the 12D:12L which was exposed to a short interval of photoperiod which was discordant with the finding of Rad et al.38 who highlighted that the length of time spent feeding is inversely related to feed conversion rates, leading to improved growth. The 12D:12L duration of photoperiod was found to be appropriate for the growth of C. gariepinus in the present study. However, this was a divergence with (16 L:8 D) photoperiod reported by Gines et al.6 for maximum growth in Gilthead sea bream. The reason for this increased growth performance and weight derivatives observed in this present study could be attributed to adaptation to the 12D:12L photoperiod over 0D:24L and 24D:L. Better feed conversion efficiency in this photoperiod regime also contributed to the increased growth recorded over other exposures. Hossain et al.39 rightly stated that light and dark alternation generally are the main synchronizer of feeding activity, this is justified in this present study.
The 12L:12D photoperiod schedule has an impact on the general outcome of C. gariepinus. Fish exhibited enhanced growth and a higher specific growth rate when compared to those kept under constant illumination. Kazemi et al.40 similarly discovered that larvae of Persian sturgeon exhibited maximum weight and length when subjected to 12L:12D, 300 lux treatment. The better growth performance under a 12L:12D photoperiod is also related to the feed consumptionability of the fish. This was in contrast to the findings of Boujard et al.41, who reported that Hoplosternum littorale (also a nocturnal fish) showed no differences in feed intake when the light period was artificially increased. The rise in feed conversion ratio observed in the catfish raised under alternating light conditions 12D:12L, compared to those raised under constant light 0D:24L and complete light restriction 24D:0L can potentially be attributed to the feeding behavior of bottom dwelling species. These species tend to forage more efficiently in dark environments, resembling their natural habitats42. Pedreira et al.24 confirmed this when they confirmed that dark environments are favourable for larval rearing of L. alexandri species with a benthic habit, also observed in other catfish43,44.
Growth performance and nutrient utilization of fish are determined by gross composition of the feed ingredients, processing and storage of the feed products45. The ability of an organism to utilize nutrients, especially protein will positively influence its growth rate46. This is justified by the highest PER (0.70±0.01) and low FCR in the 12D:12L photoperiod group in this study. This suggested that fish in the 12D:12L photoperiod group must have efficiently converted feed consumed to growth. The high specific growth rate observed in this study under 12D:12L light exposure was a result of the complete feeding and utilization of the feeding alternations in the light and dark phases, more so because these fishes are nocturnal feeders. Similar results were reported for nocturnal behavioral species, such as the African catfish and the Anguilla anguilla (Linnaeus, 1758), which had better feed conversion in dark environments47,48. The higher survival rate in the 12D:12L photoperiod regimes observed in the present study corroborated the reports of Ataguba et al.49 for LD (10.49±0.28), L (8.39±2.45) D (9.16±1.62); SGR for LD (10.49±0.28), L (8.39±2.45, D (9.16±1.62) and MFW for LD (22.5±1.5), L (21.5±1.5) and D (21.5±0.5) respectively.
On the economic parameters investigated, the result from Table 2 showed significant differences (p<0.05) for C. gariepinus exposed to different photoperiod regimes. Economic conversion ratio (ECR), ICA and cost of feed varied significantly (p<0.05). However, there were no significant differences (p>0.05) in net production value (NPV), gross profit (GP), profit index (PI) and benefit cost ratio (BC) across the different photoperiod regimes. The PI, BCR, GP, NPV were all highest in 12D:12L photoperiod regimes, while ICA and ECR were maximum in 0D:24L photoperiod group. The three different photoperiod schedules exerted separate influences on economic factors observed in this study for C. gariepinus. They interacted to affect parameters such as there was an interacting effect on profit index, net production value, investment cost analysis and benefit- cost ratio. However, 12D:12L photoperiod regime had the highest value of profit index, gross profit, net production value and benefit- cost ratio, indicating that this alternation regime is more efficient for the production of C. gariepinus than 0D:24L and 24D:0L. This result was in consonance with the submission by Ajani et al.50 on growth and economic performance of Clarias gariepinus. Feeding at 5% body weight have been found to be sufficient for maximum growth of a number of different fish species51. This generally agreed with the result of this study as depicted by the net and gross profit value obtained together with parameters already mentioned.
As 12.7 to 15.0 ug/dL was the range of cortisol levels in this present study. The highest value was observed in 0D:24L, while the least was observed in the 12D:12L photoperiod exposures. Costa et al.25 noticed an increase in observed the elevation of cortisol in L. alexandri juvenile when they were raised in low-light environments which contradicts the result of this present study. This difference may be explained by the argument that the baseline cortisol levels vary significantly among other species of catfish. Thus, the plasma cortisol concentrations observed in the present study were at basal levels for the species and after a long experimental period, the fish were adapted to daily management.
Research on blood glucose as a stress indicator in fish showed significant variability in observations, particularly during stressful conditions. Blood glucose levels either remained the same or required a longer period of stress to exhibit any alteration52. Srivastava and Choudhary52 reported that exposure of Clarias batrachus to artificial photoperiod (24L:0D and 0L:24D) for a short duration of 24 hrs did not show any significant change in blood glucose concentrations. However, in the present study, FBS (Glucose) increased as the number of light hours increases, fish exposed to 00D:24L photoperiod had the highest FBS (Glucose), while those in 24D:00L had the least. The present study therefore revealed a correlation between the three photoperiod regimes and the glucose level. The FBS (Glucose) was inversely proportional to the increased light phases, while total light regime influenced plasma cortisol levels. This present study corroborated the finding of Solomon and Okomoda53 on African catfish exposed to the same photoperiod regimes. However, low light environments improve protein (total) in this current study.
Protein (total) was highest in the 24D:0L (38.00±1.52753) and the lowest value (30.333±0.88192) was obtained in the 12D:12L photoperiod regime. The study revealed that the best protein value was obtained in the total darkness phase followed by total light phase while the alternation phase ranked least.
CONCLUSION
This study demonstrated that the growth of juvenile catfish was influenced by various photoperiod schedules, with the highest growth observed in groups exposed to12 hrs of darkness and 12 hrs of light photoperiod regimen. The 12 hrs darkness and 12 hrs light schedule of light, was deemed optimal for the healthy growth and survival of catfish due to enhanced feed intake and improved feed conversion efficiency. The current study also suggests that African catfish need a short light period during a 24 hrs cycle. This was indicated by the highest growth rate at the 12D:12L photoperiod regime. The study further revealed that glucose was inversely proportional to the increased light phases, while total light regime influenced plasma cortisol levels. The profitability of fish production on both the final weight gain and fish survival, which are influenced by the farmer's understanding of fish production and the implementation of appropriate technical management practices. For successful and profitable catfish farming, the 12 hrs of darkness and 12 hrs of light photoperiod regimen is considered the most advantageous and ideal for achieving optimal yields.
SIGNIFICANCE STATEMENT
The growth and welfare of catfish can be affected by many factors. This experiment aimed to investigate the effects of different photoperiod regimes on the growth performance, nutrient utilization, welfare and economic indices of the fish under culture. Results obtained showed favorable growth and economic indices in the 12D:12L photoperiod group. From these results, further research can be carried out. The statistics here will encourage farmers on best practices to achieve optimal harvest.
REFERENCES
- Valenzuela, A., V. Campos, F. Yañez, K. Alveal and P. Gutiérrez et al., 2012. Application of artificial photoperiod in fish: A factor that increases susceptibility to infectious diseases? Fish Physiol. Biochem., 38: 943-950.
- Saraiva, J.L., M.F. Castanheira, P. Arechavala-López, J. Volstorf and B.H. Studer, 2018. Domestication and Welfare in Farmed Fish. In: Animal Domestication, Teletchea, F. (Ed.), IntechOpen, London, United Kingdom, ISBN: 978-1-83881-133-4, pp: 109-135.
- Lancaster, K. and J. Boyd, 2015. Redefinition, differentiation, and the farm animal welfare debate. J. Appl. Commun. Res., 43: 185-202.
- Bano, F. and M. Serajuddin, 2018. Photoperiodic modulation on growth and behaviour of the giant gourami, Trichogaster fasciata (Bloch and Schneider, 1801). Turk. J. Fish. Aquat. Sci., 18: 91-100.
- Toni, M., A. Manciocco, E. Angiulli, E. Alleva, C. Cioni and S. Malavasi, 2019. Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal, 13: 161-170.
- Ginés, R., J.M. Afonso, A. Argüello, M.J. Zamorano and J.L. López, 2004. The effects of long-day photoperiod on growth, body composition and skin colour in immature gilthead sea bream (Sparus aurata L.). Aquacult. Res., 35: 1207-1212.
- Litvak, M.K., V. Zadmajid and I.A.E. Butts, 2020. Growth and survival of winter flounder (Pseudopleuronectes americanus) larvae reared on different photoperiod regimes from hatch to metamorphosis. Aquacult. Res., 51: 2314-2321.
- Saha, S., K.M. Singh and B.B.P. Gupta, 2022. Circadian rhythm of expression of core clock genes in the photosensitive pineal organ of catfish, Clarias gariepinus under different photoperiodic regimes. Biol. Rhythm Res., 53: 258-282.
- Biswas, A.K., T. Morita, G. Yoshizaki, M. Maita and T. Takeuchi, 2005. Control of reproduction in Nile tilapia Oreochromis niloticus (L.) by photoperiod manipulation. Aquaculture, 243: 229-239.
- Samuel, P.O., U. Nuraini, A.V. Ayanwale, A.Z. Muhammed, S.A. Mgbemena and Y.I. Auta, 2021. Effects of photoperiod regimes on growth performance of Heterobranchus bidorsalis (Geoffrey St. Hilaire, 1809) fingerlings under laboratory conditions. J. Aquacult. Fish., 5.
- Xu, G., Z. Yuan, J. Hou, J. Zhao, H. Liu, W. Lu and J. Wang, 2021. Prolonging photoperiod promotes testosterone synthesis of Leydig cells by directly targeting local melatonin system in rooster testes. Biol. Reprod., 105: 1317-1329.
- Simensen, L.M., T.M. Jonassen, A.K. Imsland and S.O. Stefansson, 2000. Photoperiod regulation of growth of juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Aquacalture, 190: 119-128.
- Peña, R., S. Dumas, R. Saldivar-Lucio, G. García, A. Trasviña and D. Hernández-Ceballos, 2004. The effect of light intensity on first feeding of the spotted sand bass Paralabrax maculatofasciatus (Steindachner) larvae. Aquacult. Res., 35: 345-349.
- Morro, B., P. Balseiro, A. Albalat, C. Pedrosa and S. Mackenzie et al., 2019. Effects of different photoperiod regimes on the smoltification and seawater adaptation of seawater-farmed rainbow trout (Oncorhynchus mykiss): Insights from Na+, K+-ATPase activity and transcription of osmoregulation and growth regulation genes. Aquaculture, 507: 282-292.
- Puvanendran, V. and J.A. Brown, 2002. Foraging, growth and survival of Atlantic cod larvae reared in different light intensities and photoperiods. Aquaculture, 214: 131-151.
- Valenzuela, A., I. Rodríguez, B. Schulz, R. Cortés, J. Acosta, V. Campos and S. Escobar-Aguirre, 2022. Effects of continuous light (LD24:0) modulate the expression of lysozyme, mucin and peripheral blood cells in rainbow trout. Fishes, 7.
- Hisar, S.A., B. Kirim, S. Bektas, K. Altinkaynak, O. Hisar and T. Yanik, 2005. Effect of photoperiod on plasma thyroxine hormone level of mirror carp (Cyprino carpio) raised at low water temperature in a controlled environment. Israeli J. Aquacult. Bamidgeh, 57: 19-24.
- Mustapha, M., B. Okafor, K. Olaoti and O. Oyelakin, 2012. Effects of three different photoperiods on the growth and body coloration of juvenile African catfish, Clarias gariepinus (Burchell). Arch. Pol. Fish., 20: 55-59.
- Boeuf, G. and J. Falcôn, 2001. Photoperiod and growth in fish. Vie Milieu, 51: 247-266.
- Purchase, C.F., D.L. Buyee and J.A. Brown, 2000. Growth and survival of juvenile flounder, Pleuronectes ferrugineus (Storer) under different photoperiods. Aquacult. Res., 31: 547-552.
- Wang, K., K. Li, L. Liu, C. Tanase, R. Mols and M. van der Meer, 2023. Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquacult. Fish., 8: 85-90.
- Leonardi, M.O. and A.E. Klempau, 2003. Artificial photoperiod influence on the immune system of juvenile rainbow trout (Oncorhynchus mykiss) in the Southern Hemisphere. Aquaculture, 221: 581-591.
- Lazado, C.C. and P.V. Skov, 2019. Secretory proteins in the skin mucus of Nile tilapia (Oreochromis niloticus) are modulated temporally by photoperiod and bacterial endotoxin cues. Fishes, 4.
- Pedreira, M.M., E.V. Sampaio, J.C.E. dos Santos and A.V. Pires, 2012. Larviculture of two neotropical species with different distributions in the water column in light- and dark-colored tanks. Neotrop. Ichthyol., 10: 439-444.
- Costa, D.C., C.C. Mattioli, W.S. Silva, R. Takata, F.O.P. Leme, A.L. Oliveira and R.K. Luz, 2017. The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J. Fish Biol., 90: 922-935.
- Almazán-Rueda, P., A.T.M. van Helmond, J.A.J. Verreth and J.W. Schrama, 2005. Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. J. Fish Biol., 67: 1029-1039.
- Appelbaum, S. and E. Kamler, 2000. Survival, growth, metabolism and behavior of Clarias gaeriepinus (Burchell 1822) early stages under different light conditions. Aquacult. Eng., 22: 269-287.
- Almazan-Rueda, P., A.T.M. Van Helmond, J.A.J. Verreth and J.W. Schrama, 2005. Photoperiod affects growth, behavior and stress variables in Clarias gariepinus. J. Fish Biol., 67: 1029-1039.
- Davie, A., C.M. de Quero, N. Bromage, J. Treasurer and H. Migaud, 2007. Inhibition of sexual maturation in tank reared haddock (Melanogrammus aeglefinus) through the use of constant light photoperiods. Aquaculture, 270: 379-389.
- Ofor, C.O. and O.E. Afia, 2015. Effect of stocking densities on growth and feed utilization of hybrid catfish (Clarias gariepinus x Heterobranchus longifilis) fed at 1% body weight. Am. J. Biol. Life Sci., 3: 211-217.
- Adebayo, I., 2019. Effect of photoperiod on eggs hatchability, growth and survivability of hybrid catfish (Heterobranchus bidorsalis x. Clarias gariepinus) larvae. J. Aquat. Fish., 2.
- Zhu, D., K. Yang, Y. Gul, W. Song, X. Zhang and W. Wang, 2014. Effect of photoperiod on growth and gonadal development of juvenile Topmouth Gudgeon Pseudorasbora parva. Environ. Biol. Fish., 97: 147-156.
- Mohanty, R.K., 2004. Density-dependent growth performance of Indian major carps in rainwater reservoirs. J. Appl. Ichthyol., 20: 123-127.
- Engle, C.R. and D. Valderrama, 2001. Effect of stocking density on production characteristics, costs, and risk of producing fingerling channel catfish. North Am. J. Aquacult., 63: 201-207.
- Sebastian, V., G. Davison and Lebechi, 2014. Causes of feeding frequency on feeding efficiency, growth and economic feasibility of rearing African catfish (Clarias gariepinus) juveniles and fingerlings. Adv. Aquacult. Fish. Manage., 2: 128-131.
- Migaud, H., P. Fontaine, P. Kestemont, N. Wang and J. Brun-Bellut, 2004. Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture, 241: 561-574.
- Kashyap, A., B.C. Pathak, M. Awasthi and M. Serajuddin, 2015. Effect of different photoperiods on the growth and survival of juvenile of Indian major carp, Catla catla. Iran. J. Fish. Sci., 14: 946-955.
- Rad, F., S. Bozaoğlu, S.E. Gözükara, A. Karahan and G. Kurt, 2006. Effects of different long-day photoperiods on somatic growth and gonadal development in Nile tilapia (Oreochromis niloticus L.). Aquaculture, 255: 292-300.
- Hossain, M.A.R., M.C.M. Beveridge and G.S. Haylor, 1998. The effects of density, light and shelter on the growth and survival of African catfish (Clarias gariepinus Burchell, 1822) fingerlings. Aquaculture, 160: 251-258.
- Kazemi, R., M. Yarmohammadi, A. Hallajian, J. Jalilpour and F. Esmaeili, 2020. Influence of the photoperiod and light intensity on growth performance, melatonin and insulin-like growth factors gene expression on Acipenser persicus during the embryonic stage. Iran. J. Fish. Sci., 19: 1175-1192.
- Boujard, T., Y. Moreau and P. Luquet, 1991. Entrainment of the circadian rhythm of food demand by infradian cycles of light-dark alternation in Hoplosternum littorale (Teleostei). Aquat. Living Resour., 4: 221-225.
- Veras, G.C., L.D.S. Murgas, M.G. Zangeronimo, M.M. Oliveira, P.V. Rosa and V.O. Felizardo, 2013. Biological rhythms and photoperiod in fish [In Portuguese]. Archivos Zootecnia, 62: 25-43.
- Santos, T.G., M. Schorer, J.C.E. dos Santos, A. Pelli and M.M. Pedreira, 2019. The light intensity in growth, behavior and skin pigmentation of juvenile catfish Lophiosilurus alexandri (Steindachner). Lat. Am. J. Aquat. Res., 47: 416-422.
- Assega, F.M., J.L.O. Birindelli, A. Bialetzki and O.A. Shibatta, 2016. External morphology of Lophiosilurus alexandri Steindachner, 1876 during early stages of development, and its implications for the evolution of pseudopimelodidae (siluriformes). PLoS ONE, 11.
- Oluyinka, A.A., A. Funmilola and F.O. Richards, 2015. Nutrient utilization and growth of Clarias gariepinus fed four different commercial feeds. Int. J. Fish. Aquacult., 7: 107-110.
- Sogbesan, A.O. and A.A.A. Ugwumba, 2008. Nutritional evaluation of termite (Macrotermes subhyalinus) meal as animal protein supplements in the diets of Heterobranchus longifilis (Valenciennes, 1840) fingerlings. Turk. J. Fish. Aquat. Sci., 8: 149-158.
- Rodríguez, A., F. Castelló-Orvay and E. Gisbert, 2009. Somatic growth, survival, feed utilization and starvation in European elver Anguilla anguilla (Linnaeus) under two different photoperiods. Aquacult. Res., 40: 551-557.
- Dedeke, G.A., F.O. Kehinde and I.A. George, 2023. The growth response and digestive enzyme activity of juvenile African catfish (Clarias gariepinus) exposed to artificial light at night (ALAN) spectral. Jordan J. Biol. Sci., 16: 692-698.
- Ataguba, G.A., V.T. Okomoda and V.T. Azave, 2015. Effect of photoperiod on hatching success of eggs and early fry performance of Clarias gariepinus (Siluriformes, Burchell 1822). Trakia J. Sci., 13: 171-174.
- Ajani, E.K., O. Orisasona and A. Jenyo-Oni, 2015. Growth and economic performance of Clarias gariepinus fry reared at various stocking densities. J. Fish. Livest. Prod., 3.
- Pottinger, T.G., T.R. Carrick and W.E. Yeomans, 2002. The three-spined stickleback as an environmental sentinel: Effects of stressors on whole-body physiological indices. J. Fish Biol., 61: 207-229.
- Srivastava, S. and S.K. Choudhary, 2010. Effect of artificial photoperiod on the blood cell indices of the catfish, Clarias batrachus. J. Stress Physiol. Biochem., 6: 22-32.
- Solomon, S.G. and V.T. Okomoda, 2012. Growth response and aggressive behaviour of Clarias gariepinus fingerlings reared at different photoperiods in a water re-circulatory system. Livest. Res. Rural Dev., 24.
How to Cite this paper?
APA-7 Style
Afia,
O.E., Olaseni,
M.B., Okpon,
A. (2024). Impacts of Photoperiod Manipulation on Growth and Welfare of Juvenile African Catfish. Asian Journal of Biological Sciences, 17(4), 523-534. https://doi.org/10.3923/ajbs.2024.523.534
ACS Style
Afia,
O.E.; Olaseni,
M.B.; Okpon,
A. Impacts of Photoperiod Manipulation on Growth and Welfare of Juvenile African Catfish. Asian J. Biol. Sci 2024, 17, 523-534. https://doi.org/10.3923/ajbs.2024.523.534
AMA Style
Afia
OE, Olaseni
MB, Okpon
A. Impacts of Photoperiod Manipulation on Growth and Welfare of Juvenile African Catfish. Asian Journal of Biological Sciences. 2024; 17(4): 523-534. https://doi.org/10.3923/ajbs.2024.523.534
Chicago/Turabian Style
Afia, Ofonime,, Edet, Musa, Babatunde Olaseni, and Aniekpeno Okpon.
2024. "Impacts of Photoperiod Manipulation on Growth and Welfare of Juvenile African Catfish" Asian Journal of Biological Sciences 17, no. 4: 523-534. https://doi.org/10.3923/ajbs.2024.523.534

This work is licensed under a Creative Commons Attribution 4.0 International License.



