Ameliorative Potential of Ethanol Leaves Extract of Parkia biglobosa on Acetaminophen-Induced Oxidative Stress in Albino Rats
| Received 17 Mar, 2024 |
Accepted 15 May, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Traditional medicine uses the medicinal plant, Parkia biglobosa for the therapy of several diseases. Ethanol extract of Parkia biglobosa leaves’ ability to mitigate oxidative stress induced by acetaminophen was investigated. Materials and Methods: The 25 Wistar rats were randomly divided into 5 treatment group: Group I (distilled water alone), group II (500 mg/kg b.wt., of acetaminophen alone), groups III (500 mg/kg b.wt., of acetaminophen and 140 mg/kg Silymarin), group IV (500 mg/kg b.wt., of acetaminophen and 200 mg/kg of Parkia biglobosa leaves extract) and group V (500 mg/kg b.wt., of acetaminophen and 300 mg/kg of Parkia biglobosa leaves extract). The animals were sacrificed after 21 days and the liver was isolated and biochemically analysed. Results: A significant (p<0.05) decrease in (CAT), superoxide dismutase (SOD) and Glutathione Peroxidase (GPx) activities in the acetaminophen-treated group when compared to the normal control group, whereas the extract-treated groups exhibited a substantial (p<0.05) rise in the enzymes’ activities. In addition, thiobarbituric acid reactive substances (TBARS) showed a significant (p<0.05) elevation in group II, but was markedly (p<0.05) reduced in group IV and V upon extract administration. Acetaminophen treatment caused the body weight of the rats to significantly (p<0.05) decrease when compared to the control group, but extract administration in groups IV and V restored the body weights. Conclusion: Tha Parkia biglobosa exhibited a strong antioxidant effect by ameliorating liver damage caused by acetaminophen-induced oxidative stress and this pharmacological effect may be linked to its high amount of antioxidant compounds. Parkia biglobosa leaves need to be studied further to synthesize the bioactive compounds eliciting the antioxidant properties.
| Copyright © 2024 Osagie et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Acetaminophen, often known as paracetamol, is a frequently utilized drug that alleviates pain and lowers body temperature. It is known to induce oxidative stress, leading to liver damage and other health problems. Increased consumption of paracetamol, whether intentional or accidental, can cause severe liver damage due to the increased production of harmful free radicals and the decrease in protective antioxidants in the liver tissue. This is a widely recognized experimental paradigm for studying liver toxicity1. While therapeutic dosages of this drug are considered safe, an overdose can lead to significant liver damage known as hepatic necrosis and hepatic failure2. The pathophysiology of paracetamol-induced liver injury involves the activation of cytochrome P450 (CYP450) isoforms (CYP2E1, CYP3A4 and CYP1A2), depletion of intracellular glutathione and oxidative stress. The production of N-acetyl-P-benzoquinone imine (NAPQI), a reactive metabolite, is primarily responsible for these activities. The NAPQI is generated during the oxidation of acetaminophen, catalysed by cytochrome P4502. Acetaminophen-induced liver damage primarily stems from oxidative stress, an imbalance between reactive oxygen species and antioxidant defense systems. This is primarily due to the occurrence of lipid peroxidation, a reduction in antioxidant enzymes and an impairment of mitochondrial function3. Therefore, it is crucial to discover efficacious therapies to alleviate the oxidative stress caused by acetaminophen.
Various countries, including affluent nations, widely use traditional medicine for healthcare4. Scientists have concentrated their efforts on studying the bioactive substances derived from plants used in herbal medicine. This is due to bacteria’s increasing resistance and negative effects against antibiotics5. These plants’ medical importance stems from the chemical compounds they possess, which have distinct physiological effects on the human body6. To explore new treatment methods for certain conditions like cancer7 and metabolic inflammation8, scientists have focused their efforts on plant phytochemicals. For an extended period, the examination of medicinal plant components to determine their therapeutic potential has consistently been an avenue for discovering potent novel drugs9.
Phytochemicals include a variety of natural products found in plants that have therapeutic qualities. These substances, such as polyphenols and carotenoids, can prevent or slow down the oxidation of lipids and other molecules by stopping the start or spread of oxidative chain reactions10. Parkia biglobosa, commonly referred to as the African locust bean, is a plant extensively employed in West Africa for its medicinal properties. Traditional medicine in tropical Africa frequently advocates its use for a diverse range of health advantages. People prepare a decoction from the plant’s bark, root and leaves to treat fevers, hypertension and toothaches11. Prior studies have established the phenolic components12 and hypotensive effects of the leaf extract13. However, the leaf’s antioxidant activity in acetaminophen-induced oxidative stress is not well understood. The purpose of this study was to examine the ability of ethanol leaf extract from Parkia biglobosa to ameliorate acetaminophen-induced oxidative stress in albino rats in order to give insight to its possible therapeutic potential and usage in the treatment of oxidative stress-related diseases.
MATERIALS AND METHODS
Study location: The study was carried out at the Department of Biochemistry, Federal University Wukari, Nigeria from September, 2022 to January, 2023.
Plant material collection: Fresh Parkia biglobosa leaves were obtained opposite Federal University, located in Wukari, Taraba State, Nigeria. The foliage was cleansed by rinsing it in clean water to get rid of any dirt or dust, allowed to dry in the shade for 2 weeks and then ground in a mill.
Preparation of plant extract: The preparation was carried out as described by Ale et al.14. The 2000 g of the pulverized leaves were dissolved into a jar containing 2 L of absolute ethanol. The mixture was macerated with continuous stirring periodically for 72 hrs. After which the solvent was filtered using muslin cloths followed by Whatman filter paper. Using a rotary vacuum evaporator RE52, the ethanol was extracted from the filtrate and the residues were then collected and employed in the experiment.
Experimental animals: The 25 Wistar rats (male and female) having an average body weight of 170 g were purchased from Yola, Adamawa State, Nigeria and tended to in the Biochemistry Department animal house at Federal University Wukari. They were housed in clean cages with litter to keep them warm. They received pellet-based food and were managed in accordance with the guidelines established by the Federal University Wukari, Nigeria’s Committee on Care and Use of Experimental Animal Resources, with approval number FUW/FPAS/23/017. They were given 2 weeks to acclimatize.
Experimental design: The 25 Wistar rats were divided into 5 groups using a randomized block design (n = 5); animals in group I served as the control group, they were given feed and distilled water only with no treatment; group II (negative control), were given acetaminophen 500 mg/kg b.wt., daily, feed and distilled water without treatment, group III (positive control), were given acetaminophen, single dose of 500 mg/kg daily and treated with standard drug, silymarin (140 mg/kg ), group IV and V were given acetaminophen, single dose of 500 mg/kg daily and treated with Parkia biglobosa ethanol extract (200 and 300 mg/kg, respectively). Treatment was administered orally using oral gavage for 21 days. Under chloroform anesthesia, all animals were slaughtered 24 hrs after the final dose of the extract was administered and the organs were promptly removed, quickly cleaned in ice-cold normal saline and preserved at -20oC for analysis of MDA, GPx, TBARS, superoxide dismutase (SOD) and catalase (CAT) activities, in order to ascertain the antioxidant activity of the plant leaves.
Liver homogenates preparation: To obtain 10% (w/v) homogenates, 1 g of liver tissues were homogenized in 10 mL of ice-cold physiological saline. After centrifuging the resulting homogenates at 5,000 rpm for a period of 10 min, the supernatants were obtained and the activity of superoxide dismutase (SOD), catalase (CAT), MDA, GPx and TBARS was assessed.
In vivo antioxidant study
Superoxide dismutase (SOD) activity: The technique outlined by Martin et al.15 was used to test the superoxide dismutase activity. The test material, a tissue homogenate, was combined with 920 μL of phosphate buffer (0.05 M, pH 7.8) in a sterile test tube. Additionally, a reagent test was conducted by introducing 40 μL of assay buffer (phosphate buffer pH 7.8, 0.05 M) into a separate, uncontaminated test tube. After agitating, the combinations were allowed to rest at ambient temperature for 2 min. In a clean test tube, 920 μL of phosphate buffer (0.05 M, pH 7.8) was added to the sample (tissue homogenate), which was utilized as the test sample. Another clean test tube was filled with 40 μL of assay buffer (phosphate buffer pH 7.8, 0.05 M) to provide a reagent test (blank/without sample). The mixtures were shaken and then allowed to stand for 2 min at room temperature. Additionally, 40 μL of hematoxylin was swiftly combined into the blank test tubes for the reagent and sample, respectively, to initiate the auto-oxidation reaction. The sample and reagent absorbance test was carried out following the addition of 40 μL of hematoxylin:
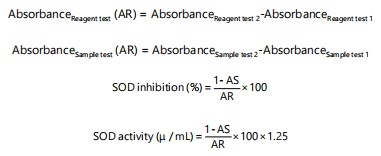 |
where, AS/AR is the ratio of the difference between the sample and the reference absorbance.
Catalase (CAT) activity: The methodology outlined by Aebi16 was employed to quantify catalase activity. After adding a working solution (consisting of 50 mM potassium phosphate buffer with a pH of 7.0, in a volume of 1000 μL) to a cuvette, the spectrophotometer (Shimadzu UV-1800, Mumbai, Maharashtra, 400067, India) was calibrated at a specific wavelength of 240 nm. In addition, 950 μL of the functional buffer (490 μL of 50 mM potassium phosphate buffer, pH 7.0), 460 μL of 30 mM hydrogen peroxide (H2O2) and 50 μL of a sample (tissue homogenate) were put into a separate clean cuvette and quickly mixed.
In order to create a catalase standard, 950 μL of working assay buffer were combined with 50 μL of diluted catalase standard. Every minute for 5 min, at 240 nm, the rate of H2O2 oxidation was determined. The sample’s deterioration rate was recorded as (A240 nm/min), while the catalase activity was determined and presented as (μ/mL):
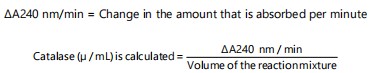 |
Activity of glutathione peroxidase (GPx) glutathione: Paglia and Valentine17 improved approach was used for the peroxidase assay. The samples (tissue homogenate) were placed on ice and all reagents were prepared and used at room temperature. The glutathione reductase and NADPH diluent (4 mM NaN3 in buffer with stabilizer) were used to reassemble the NADPH reagent (-nicotinamide adenine dinucleotide phosphate and GSH reduced). The 50 μL of working NADPH was added and then precisely 50 μL of the sample was transferred to a clean test tube. Also, the sample test tube received 50 μL of working H2O2 (0.3 mL of 3% H2O2 diluted to 10 mL with assay buffer), which was then given a minute to adjust. The sample was removed from the tube and 50 mL of distilled water was added to create the blank tube. The mixtures from both tubes were transferred to cuvettes and the absorbance was measured for 5 min at 340 nm while comparing each reading to a blank sample every 30 sec. The net rate was used to compute the glutathione peroxidase activity, which was then represented as (μ/mL):
where, mRates is the measurement of the sample rates and mRate b is the measurement of the blank rates.
Thiobarbituric acid reactive substances (TBARS): Using the method outlined by Fraga et al.18, in the tissues, the presence of thiobarbituric acid reactive compounds (TBARS) was identified. A clean sample centrifuge tube was used to mix the 250 μL of tissue homogenate (the sample), 250 μL of the acid reagent (1 M phosphoric acid), 10 μL of the BHT reagent (10 μL of butylated hydroxytoluene in ethanol) and 250 μL of the TBA reagent (2 mL of 2-thiobarbituric acid restored with 10.5 mL distilled water) were used. By substituting 250 μL of distilled water fr the sample, a blank test was obtained. Following that, at 60°C in a water bath, the tubes were incubated for 60 min. After cooling, it underwent a 3 min, 10,000×g centrifugation. After that, cuvettes were used to hold the reaction mixture in both tubes and measured for 5 min against a blank sample using an absorbance reading at 532 nm. The unit of measurement for TBARS concentration is the malondialdehyde (MDA)corresponds to (μM).
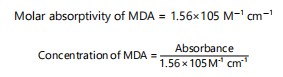 |
Statistical analysis: Version 20 of the Statistical Package for Social Sciences (SPSS) was used for Analysis of Variance (ANOVA) for variation in treatment means and a Duncan’s multiple comparisons test on all data. The data was presented as Mean±Standard Deviation (n = 5), result means were compared for statistical significance (p<0.05).
RESULTS
Effect of Parki biglobosa on glutathione peroxidase (GPx) activity: Table 1 reveals that group IV has the highest GPx activity, followed by group V, while group II treated with acetaminophen alone shows a considerable reduction compared with the normal control group.
| Table 1: | Effect of Parkia biglobosa leaves ethanolic extract on antioxidant enzymes in the liver homogenate of acetaminophen (Paracetamol PCM)-induced hepatotoxicity in Wistar rats | |||
| Treatment groups | CAT | SOD | GPx | TBARS |
| I Normal control (distilled water) | 0.88±0.05ab | 150.00±5.64b | 16.21±1.22b | 1.37±0.12ab |
| II Negative control (PCM) | 0.53±0.03a | 78.64±2.33a | 9.10±2.05ba | 2.84±0.25d |
| III Positive control (PCM+silymarin) | 1.04±0.04b | 221.36±5.42cd | 24.88±2.55c | 1.52±0.04b |
| IV PCM+200 mg/kg b.w., extract | 2.12±0.08c | 193.63±7.67c | 27.00±0.05d | 1.18±0.14a |
| V PCM+300 mg/kg b.w., extract | 1.78±0.13bc | 245.45±6.65d | 25.23±0.09cd | 1.79±0.15c |
| Values are presented as Mean±Standard Deviation. Within the same column, different superscripted values differ considerably at p<0.05. CAT: Catalase (U/mg protein/min), SOD: Superoxide dismutase (U/mg protein/min), GPx: Glutathione peroxidase (U/mg protein/min) and TBARS: Thiobarbituric acid reactive substances (U/mg protein/min) | ||||
| Table 2: | Effect of Parkia biglobosa leaf ethanolic extract on Wistar rats’ body weight after acetaminophen (paracetamol)-induced liver toxicity | |||
| Treatment groups | Week one (g) | Week two (g) | Week three (g) |
| I Normal control (distilled water) | 146.40±5.59a | 173.80±5.86a | 188.60±5.75a |
| II Negative control (PCM) | 169.00±11.73b | 145.40±10.11c | 121.00±8.46b |
| III Positive control (PCM+silymarin) | 168.20±9.29b | 176.80±8.41a | 196.80±3.93ab |
| IV PCM+200 mg/kg b.w., extract | 155.40±10.22ab | 162.20±10.63b | 177.00±10.18c |
| V PCM+300 mg/kg b.w., extract | 153.60±8.87ab | 181.60±9.56ab | 204.80±7.29d |
| Values are presented as Mean±Standard Deviation. Within the same column, different superscripted values differ considerably at p<0.05 | |||
Effect of Parki biglobosa on superoxide dismutase (SOD) activity: Comparing group II, which received 500 mg/kg of acetaminophen without therapy, to group I, the SOD level dropped significantly. On the other hand, group VI and V exhibited an increase in SOD activity comparable to conventional medication in the third group. However, 300 mg/kg was more effective than 200 mg/kg (Table 1).
Effect of Parki biglobosa on catalase (CAT) activity: As shown in Table 1, groups IV and V have the highest catalase activity followed by group III, while the lowest activity was recorded in group II.
Effect of Parki biglobosa on TBARS activity: The results in Table 1 reveal that group II has the highest TBARS activity, while Groups IV and V produced TBAR levels that are significantly lower (p<0.05) than group II.
Effect of Parki biglobosa on animal weight: When compared to group I, group II, which received 500 mg/kg of acetaminophen without treatment over 21 days, there was a substantial (p<0.05) weight loss. The conventional medicine (group III) elicited an effect comparable to the weight gain seen in group IV and V, which is significant as shown in Table 2.
DISCUSSION
Free radicals and antioxidants are in an unfavorable balance known as oxidative stress, which can impair redox signaling and regulation and/or induce molecular damage. One standard hallmark of the toxicity of substances, such as N-acetyl-para-benzoquinoneimine (NAPQI), an active metabolite of acetaminophen, is the increased reactive oxygen species (ROS) production, leading to oxidative stress in cells3. Insufficient antioxidant enzyme activity leads to increased lipid peroxidation19. Glutathione is a crucial compound for the regulation of many cellular activities. It serves as a first hand antioxidant by superoxide reaction, peroxyl and single-oxygen molecules to form oxidized glutathione and other disulfides. The cells create reactive oxygen species, particularly superoxide anions and related compounds. One of these products, the hydroxyl radical is highly destructive and responsive, causing the peroxidation of lipids in cell membranes20. Malondialdehyde (MDA) is a byproduct of lipid peroxides that are produced when oxygen free radicals trigger the oxidation of lipids in tissues. Lipid peroxidation is a process where polyunsaturated fatty acids undergo oxidative destruction. This process has been associated with changes in membrane structure and the inactivation of enzymes. The significant decrease in endogenous antioxidants and the increase in MDA are indicators of acetaminophen’s oxidative effects on rat liver and blood. Lipid peroxidation, a complicated process, compromises the functionality and structure of cells. The peroxidation of the lipids in the cell membrane, which begins the process of the membrane’s integrity being destroyed, causes cell lyses. However, due to the lower activity of tissue antioxidant enzymes, lipid peroxides or protein carbonyls are more likely to cause tissue damage19.
The body produces a variety of antioxidants, such as CAT, SOD, GSH and GPx, to’mop up’ or neutralize free radicals that can harm cells and so defend themselves from oxidative stress. The body’s ability to create these antioxidants is determined by genetic makeup and environmental factors, including nutrition and chemical exposure21. The findings support the hypothesis that acetaminophen toxicity is caused in part by the antioxidant defense system’s depletion. In experimental models and people, the routines of regularly used chemotherapy typically lead to an increase in the production of free radicals and a decrease in antioxidant enzyme activities, as demonstrated by acetaminophen-induced oxidative stress22. In group II, which received 500 mg/kg of acetaminophen without treatment, there was a substantial (p<0.05) decline in levels of GPx, SOD and CAT in comparison to group I (normal control).
Additionally, there was a considerable (p<0.05) increase in the formation of TBARS in group II compared to normal control and treatment groups III, IV and V, which receive silymarin at 140, 200 and 300 mg/kg b.wt., dosages, respectively. This might be caused by low GSH levels, which boosted lipid peroxidation and led to high levels of TBARS production brought on by acetaminophen use, which in turn increased GSH consumption. Reduced glutathione, the enzyme’s substrate, may be becoming less available, which would explain the observed decline in GPx activity19. Lipid peroxidation may have developed and spread as a result of the drop in SOD activity23. Due to the decreased SOD activity, the superoxide anion produced during normal metabolism cannot be eliminated24. The increased synthesis of reactive oxygen species such superoxide and hydrogen peroxide, which in turn causes the inhibition of these enzymes’ activities, could be the cause of the decreased SOD and catalase activity19.
The ROS formation that is greater than the antioxidant system’s capacity can result in oxidative stress and damage to lipids, proteins, cells and nucleic acids19. Catalase scavenges H2O2 generated either by SOD or by free radicals during the process of removing superoxide anions. In groups IV and V of Wistar rats treated with P. biglobosa at 200 and 300 mg/kg body weight dosages in conjunction with acetaminophen, the decrease in SOD, CAT and GPx activity shown in group II rats was reversed with evidence of a considerable (p<0.05) increase in the enzymes’ activities (Table 1). In addition, the formation of TBARS adduct was markedly (P<0.05) mitigated by the extracts. It’s possible that P. biglobosa protective qualities are enhanced by the presence of polyphenols. According to Ajaiyeoba25, in addition to helping the body rid itself of xenobiotics, polyphenols also affect the expression of an important enzyme involved in cellular antioxidant defenses. Because of the significant recovery of plasma and hepatic antioxidant levels, P. biglobosa may be an efficient chemopreventive drug against oxidative stress and may lessen the acetaminophen-induced hepatic oxidative damage in rats26.
According to studies by Ogunyinka et al.27, P. biglobosa decreased oxidative stress in the liver of streptozotocin-induced diabetic Wistar rats and the result was in line with their discoveries. Strong antioxidant properties of the extract may have reduced oxygen radical production by white blood cells and enhanced cardiovascular health. Parkia biglobosa has considerable antioxidant activity and a large concentration of phenolic compounds28. Rats administered acetaminophen exhibited a substantial decrease in body weight as compared to the control group. In contrast, administration of P. biglobosa to groups IV and V resulted in a significant increase in body weight compared to the control group (group III in Table 2). This is proof of P. biglobosa effectiveness in reducing the anomalies and oxidaitive attacks brought on by acetaminophen.
Findings herein may aid in the creation of natural remedies or supplements to enhance health outcomes, especially in populations with high rates of oxidative stress-related ailments. Examining the processes behind P. biglobosa’s ability to reduce oxidative stress can throw light on the particular antioxidants and phytochemicals that give it its medicinal qualities. This may result in the discovery of novel compounds with possible uses in medicine. Additional investigation is required utilizing human models to comprehend the fundamental mechanisms of action and the particular routes.
CONCLUSION AND RECOMMENDATION
This research reveals that Parkia biglobosa leaves ethanol extract mitigated acetaminophen-induced oxidative stress in rats, by profoundly diminishing MDA levels and elevating GPx, SOD and CAT levels. Parkia biglobosa leaves may be taken into consideration for the management of diseases linked to oxidative stress because phytochemicals with antioxidant activity are able to inhibit the action of free radicals involved in the pathogenesis of conditions like cancer, atherosclerosis, liver disorders and neurodegenerative diseases. Parkia biglobosa leaves need to be studied further to synthesize the bioactive compounds exhibiting antioxidant properties and to understand the underlying mechanisms of action of the specific pathways employed.
SIGNIFICANCE STATEMENT
Oxidative stress is often implicated in several ailments and diseases including cancer, neurodegenerative diseases and cardiovascular diseases. In addition, the use of synthetic conventional drugs is usually accompanied by many disadvantages including side effects and high cost of affordability. There is therefore a need for a safer, more effective and cheaper source of therapy from plants. Meanwhile, studies have given insight into the bioactive compositions of Parkia biglobosa including phenolic compounds and flavonoids. This necessitated this research to investigate the ameliorative potential of the ethanol leaf extract of Parkia biglobosa on acetaminophen-induced oxidative stress in albino rats. Unravelling the effect of Parkia biglobosa on antioxidant indices is critical to identifying and characterizing plant’s bioactive compounds’ and could give a direction into its therapeutic use in addressing oxidative stress-related conditions.
REFERENCES
- Lebda, M.A., N.M. Taha, M.A. Korshom, A.E.W.A Mandour and R.I. Goda, 2013. Ginger (Zingiber officinale) potentiate paracetamol induced chronic hepatotoxicity in rats. J. Med. Plants Res., 7: 3164-3170.
- Bessems, J.G.M. and N.P.E. Vermeulen, 2001. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit. Rev. Toxicol., 31: 55-138.
- Jaeschke, H., M.R. McGill and A. Ramachandran, 2012. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev., 44: 88-106.
- Bursal, E., A. Aras, M. Doğru and Ö. Kiliç, 2022. Phenolic content, antioxidant potentials of Saponaria prostrata endemic plant. Int. J. Life Sci. Biotechnol., 5: 1-8.
- Arowora, K.A., O.J. Yakubu, C. Shaibu, T.J. Iornenge and K.C. Ugwuoke, 2019. Chemical composition of baobab leaves and fractionation of its ethanolic extract using column chromatography. Int. J. Sci. Res., 8: 812-821.
- Ale, E.M., S.O. Asuelimen, I.J. Umaru, B.O. Olorundare, V.I. Ayo, M.J. Timothy and P. Shadrach, 2023. Ethanolic extract of Abrus precatorius leaves protected against mercury chloride-induced hepatocellular damage and cerebral Na+/K+-ATPase dysfunction in Wistar rats. Toxicol. Adv., 5.
- Pang, S., M. Jia, J. Gao, X. Liu, W. Guo and H. Zhang, 2021. Effects of dietary patterns combined with dietary phytochemicals on breast cancer metastasis. Life Sci., 264.
- Kong, M., K. Xie, M. Lv, J. Li and J. Yao et al., 2021. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother., 133.
- Dhanasekaran, J.J. and M. Ganapathy, 2011. Hepatoprotective effect of Cassia auriculata L. leaf extract on carbon tetrachloride intoxicated liver damage in wister albino rats. Asian J. Biochem., 6: 104-112.
- Pandohee, J., E. Kyereh, S. Kulshrestha, B. Xu and M.F. Mahomoodally, 2023. Review of the recent developments in metabolomics-based phytochemical research. Crit. Rev. Food Sci. Nutr., 63: 3734-3749.
- Komolafe, K., M.T. Olaleye, T.I. Fasan, O.O. Elekofehinti, J.A. Saliu and A.A. Akindahunsi, 2013. Lipid-lowering effect of Parkia biglobosa leaf saponins in triton-X 1339-induced hyperlipidemic rats. Res. J. Pharm. Biol. Chem. Sci., 4: 576-585.
- Komolafe, K., T.M. Olaleye, O.I. Omotuyi, A.A. Boligon, M.L. Athayde, A.A. Akindahunsi and J.B.T. da Rocha, 2014. In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J. Acupuncture Meridian Stud., 7: 202-210.
- Olaleye, T.M., K. Komolafe and A.A. Akindahunsi, 2013. Effect of methanolic leaf extract of Parkia biglobosa on some biochemical indices and hemodynamic parameters in rats. J. Chem. Pharm. Res., 5: 213-220.
- Ale, E.M., A.O. Adeleye, O.R. Akinseye and E.K. Toluwalase, 2021. Antioxidant activities of ethanolic extract of Annona muricata against different pro-oxidant induced lipid peroxidation in rat brain and liver. Pharm. Pharmacol. Int. J., 9: 45-49.
- Martin, Jr. J.P., M. Dailey and E. Sugarman, 1987. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch. Biochem. Biophys., 255: 329-336.
- Aebi, H., 1974. Catalase. In: Methods of Enzymatic Analysis, Bergneyer H. (Ed.)., Academic Press, Weinham, New York, pp: 673-684.
- Paglia, D.E. and W.N. Valentine, 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med., 70: 158-169.
- Fraga, C.G., B.E. Leibovitz and A.L. Toppel, 1988. Lipid peroxidation measured as tbars in tissue slices: Characterisation and comparison with homogenate and microsome. Free Radical Biol. Med., 4: 155-161.
- Airaodion, A.I., E.O. Ogbuagu, U. Ogbuagu, A.R. Adeniji, A.P. Agunbiade and E.O. Airaodion, 2019. Hepatoprotective effect of Parkia biglobosa on acute ethanol-induced oxidative stress in Wistar rats. Int. Res. J. Gastroenterol. Hepatol., 2: 18-28.
- Musara, C., E.B. Aladejana, S.M. Mudyiwa and C. Karavina, 2020. Parkia biglobosa (Mimosaceae): Botany, uses, phytochemical properties and pharmacological potential. J. Pharm. Nutr. Sci., 10: 101-115.
- Oyewole, I., G. Anyasor, K. Ogunwenmo and S. Ayodele, 2011. Antioxidant and oxidative stress status in human Plasmodium malaria. Der Pharm. Lett., 3: 91-96.
- Du, K., A. Ramachandran and H. Jaeschke, 2016. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol., 10: 148-156.
- Akwaji, P.I., E.O. Eyam and R.A. Bassey, 2017. Ethnobotanical survey of commonly used medicinal plants in Northern Cross River State, Nigeria. World Sci. News, 70: 140-157.
- Bayala, J., J. Sanou, Z. Teklehaimanot, A. Kalinganire and S.J. Ouédraogo, 2014. Parklands for buffering climate risk and sustaining agricultural production in the Sahel of West Africa. Curr. Opin. Environ. Sustainability, 6: 28-34.
- Ajaiyeoba, E.O., 2002. Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. Afr. J. Biomed. Res., 5: 125-129.
- Ahmad, N.I., S.A. Rahman, Y.H. Leong and N.H. Azizul, 2019. A review on the phytochemicals of Parkia speciosa, stinky beans as potential phytomedicine. J. Food Sci. Nutr. Res., 2: 151-173.
- Ogunyinka, B.I., B.E. Oyinloye, F.O. Osunsanmi, A.R. Opoku and A.P. Kappo, 2016. Modulatory influence of Parkia biglobosa protein isolate on testosterone and biomarkers of oxidative stress in brain and testes of streptozotocin induced diabetic rats. Int. J. Physiol. Pathol. Pharmacol., 8: 78-86.
- Tamfu, A.N., N. Roland, A.M. Mfifen, S. Kucukaydin and M. Gaye et al., 2021. Phenolic composition, antioxidant and enzyme inhibitory activities of Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth. Arabian J. Chem. 15.
How to Cite this paper?
APA-7 Style
Osagie,
A.S., Morayo,
A.E., Joy,
T.M., Kayode,
T.E., Adewole,
M.A. (2024). Ameliorative Potential of Ethanol Leaves Extract of Parkia biglobosa on Acetaminophen-Induced Oxidative Stress in Albino Rats. Asian Journal of Biological Sciences, 17(4), 514-522. https://doi.org/10.3923/ajbs.2024.514.522
ACS Style
Osagie,
A.S.; Morayo,
A.E.; Joy,
T.M.; Kayode,
T.E.; Adewole,
M.A. Ameliorative Potential of Ethanol Leaves Extract of Parkia biglobosa on Acetaminophen-Induced Oxidative Stress in Albino Rats. Asian J. Biol. Sci 2024, 17, 514-522. https://doi.org/10.3923/ajbs.2024.514.522
AMA Style
Osagie
AS, Morayo
AE, Joy
TM, Kayode
TE, Adewole
MA. Ameliorative Potential of Ethanol Leaves Extract of Parkia biglobosa on Acetaminophen-Induced Oxidative Stress in Albino Rats. Asian Journal of Biological Sciences. 2024; 17(4): 514-522. https://doi.org/10.3923/ajbs.2024.514.522
Chicago/Turabian Style
Osagie, Asuelimen,, Steve, Ale, Ebenezer Morayo, Timothy, Mgbede Joy, Toluwalase, Ebenezer Kayode, and Mulikat Adenike Adewole.
2024. "Ameliorative Potential of Ethanol Leaves Extract of Parkia biglobosa on Acetaminophen-Induced Oxidative Stress in Albino Rats" Asian Journal of Biological Sciences 17, no. 4: 514-522. https://doi.org/10.3923/ajbs.2024.514.522

This work is licensed under a Creative Commons Attribution 4.0 International License.



