Effects of Reused Vegetable Cooking Oil by Fast Food Vendors in Abakaliki Metropolis on Brain Neurotransmitters in Normal Albino Rats
| Received 18 Mar, 2024 |
Accepted 20 Jun, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Repeatedly using vegetable cooking oils for deep-frying can cause the fats and oils to undergo oxidative degradation, resulting in the release of free radicals. The oxidation initiated by these free radicals is a significant factor leading to rancidity in fats, oils and oxidative stress in biological systems. This study aimed to explore the impact of vegetable oil reused by fast food vendors in Abakaliki Metropolis on brain neurotransmitters in normal albino rats. Materials and Methods: Thirty-five albino Wistar rats were included in this study and randomly divided into five experimental groups (A-E), with each group consisting of seven rats. Before starting the experiment, the rats were given seven days to acclimate to the conditions. The treatment period lasted for 42 days, after which brain samples were collected from the rats for laboratory analysis. Standard methods were employed to analyze the levels of serotonin, dopamine, nor-epinephrine, acetylcholinesterase (AChE) and epinephrine in the collected brain samples. Results: The results showed reductions in the amount of nor-epinephrine, dopamine and serotonin significantly (p<0.05) in rats that received reused vegetable oil (RVO) when compared to groups that received fresh vegetable oil and normal control. Noticeable increases (p<0.05) were observed in the levels of epinephrine and the activity of AChE in rats administered with RVO. The albino rats given RVO and fresh vegetable oil (FVO) exhibited a notable and statistically significant (p<0.05) increase in their body weight when compared to the control group. This weight gain was observed in a time-dependent manner. Conclusion: According to the results obtained from this research, the intake of reused vegetable oil may pose risks to the brain's well-being.
| Copyright © 2024 Asouzu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
One of the crucial nutrients consumed daily in meals is vegetable oil. Reusing oil is a frequent practice since it lowers the cost of preparing food. However, subjecting vegetable oil to repeated heating, which leads to oxidation of lipids, can diminish the benefits it offers. It is now commonly accepted that the pathogenesis of numerous harmful health impacts, including stress-associated illness, anxiety, hypertension, cardiovascular problems etc., is intimately tied to the quality of dietary oils and fats. Fried dishes are commonly enjoyed across various eating establishments in Nigeria, including restaurants, market stalls and roadside cafes. The dietary preferences of individuals are often shaped by their socioeconomic status. As an example, individuals with limited income in Ebonyi State depend on fried food sold by roadside vendors as a means of sustenance. A survey indicated that 48% of respondents consumed fried dishes between one to six times weekly1. Snacks constitute at least twenty percent of all meals, with shallow and deep-fried options emerging as the most favored categories2.
Repeatedly heating oils at high temperatures (160-190°C) for an extended duration accelerates the formation of polymerized, as well as oxidized lipid compounds in the frying medium. This process makes the oil more susceptible to polymerization, thermal oxidation and hydrolysis leading to a configuration change of fatty acids from cis to trans isomers. Repeated heating alters the oil’s physical characteristics, making it unhealthy for human ingestion by increasing its foaming, viscosity, darkening in color and lowering its smoke point. The consumption of reused vegetable oil has been linked to a range of unfavorable health effects, for example, heightened blood pressure3-6, an elevated risk of cardiovascular diseases7,8, endothelial dysfunction9, impaired vaso-relaxation responses10, hypertension11, increased lipid peroxidation and low-density lipoprotein levels12,13 as well as atherosclerosis14.
One of the most popular and traditional ways to prepare meals is deep frying. Both heat and mass are transferred. The oils are often used repeatedly for frying in order to cut costs. Subjecting the oil to repeated heating will induce alterations in its physical properties, leading to heightened viscosity and a darker color15. These changes could potentially impact the oil's fatty acid composition. Oil undergoes a number of chemical processes when heated, including polymerization, hydrolysis and oxidation16. During the frying process, a multitude of oxidative substances are formed, like aldehydes as well as, hydroperoxides that is being consumed when eating the fried food17. As a result of the heating, the oils experience a decrease in vitamin E content and lose their advantageous properties. Tocopherols are known to be abundant in vegetable oils18,19. In vegetable oils, alongside tocopherols, there exists a notable presence of tocotrienols. Studies have consistently shown that tocopherols exhibit superior antioxidant activity compared to tocotrienols20. Without considering the potential health effects, the reuse of vegetable oil in the production of fast food is becoming a regular practice among fast food vendors. The results of the study “effect of reused vegetable oil (RVO) by fast food vendors in Abakaliki Metropolis on some brain neurotransmitters in normal albino rats” will provide the baseline data that will help the policy makers in the food, nutrition and health sectors to come up with better policies to tamp down the excess of fast food vendors and the general public in general in preparing quality food. It is essential to comprehend the potential adverse effects of thermal changes that occur in oil during frying to evaluate the safety, as well as quality of food products during processes involving high temperatures or deep frying. Such understanding plays a vital role in safeguarding consumer health. Therefore, this study investigated the effect of reused vegetable cooking oil by fast food vendors in Abakaliki Metropolis on brain neurotransmitters in normal albino rats.
MATERIALS AND METHODS
Study area: The study was done in the Department of Biochemistry, Ebonyi State University, Nigeria. It was done on October, 2020.
Chemicals and materials: Analytical-grade chemicals and reagents were utilized in this study. The chemicals were obtained from reputable suppliers, including May and Baker in England, BDH in England and Merck in Darmstadt, Germany. The reagents consisted of commercial kits from Randox and QCA in the USA. Fresh vegetable oil was purchased from International Market Abakaliki, Ebonyi State while reused oil were collected from four different fast food vendors in Abakaliki Metropolis.
Methods
Experimental animals: The experimental rats were procured from the Animal House of the Faculty of Veterinary Medicine at the University of Nigeria, Nsukka, Enugu, Nigeria. These rats were housed in stainless steel cages within a well-ventilated animal house under appropriate laboratory conditions, which included a 12 hrs light/dark cycle and room temperature. They were given a period of seven days to acclimate to their new environment. Throughout the study, the rats had unrestricted access to standard rodent chow (Vital feed®, Grand Cereals Ltd., Jos, Nigeria) and water.
Ethical consideration: All experimental procedures were conducted in accordance with the guidelines outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80 23, revised in 1996). The study was granted approval by the Department of Biochemistry Ethical Review Committee at Ebonyi State University, Abakaliki, Nigeria, under the reference number EBSU/BCH/ET/20/007 on the 22nd of October, 2022.
Experimental design: For this research, thirty-five albino Wistar rats were utilized. These rats were randomly divided into five experimental groups named A, B, C, D and E, with seven rats allocated to each group. Among these groups, A and B served as control groups, while C, D and E were designated as the treatment groups:
| • | Group A: Rats received normal rats feed, water and served as the normal control | |
| • | Group B: Rats received 5 mL/kg b.wt., of fresh vegetable oil (FVO) orally | |
| • | Group C: Rats received 5 mL/kg b.wt., of reused vegetable oil (RVO) orally | |
| • | Group D: Rats received 2.5 mL/kg b.wt., of reused vegetable oil (RVO) orally | |
| • | Group E: Rats received 1.5 mL/kg b.wt., of reused vegetable oil (RVO) orally |
All the rats in the five groups received normal feed, water and the study lasted for 42 days.
Rat body weights were recorded every 7 days during the study period. After 42 days of treatment, the rats were subjected to an overnight fast and then anesthetized with ether prior to being euthanized. The brain was isolated, washed in cold saline water and dried. Next, the brain tissue was homogenized using 0.1 M phosphate buffered saline at a pH of 6.4, at a ratio of 1:5 (w/v) and the resulting homogenate was centrifuged at 4000 g for 20 min. The supernatants obtained from this process were used for the analysis of acetylcholine esterase activity and neurotransmitters.
Biochemical analyses
Determination of acetylcholinesterase: The acetylcholinesterase activity was assessed following the procedure outlined by Ellman et al21. The tissue samples were prepared by homogenizing them in 0.1M phosphate buffer at pH 7.5 and then subjected to centrifugation at 14,000 rpm for 5 min. Into distinct wells of a 96-well plate with a clear bottom, 200 μL of water and 200 μL of a calibrator were added separately. Additionally, 10 μL of the sample was introduced into two separate wells. Next, to each sample well, 190 μL of freshly prepared working reagent was introduced and the plate was gently tapped to ensure thorough mixing. The optical density was read at 2 and 10 min using a plate reader with a wavelength of 412 nm.
Calculation:
The OD10 and OD2 represent the sample’s optical density values at 412 nm measured at 2 and 10 min, respectively. The ODCAL and ODH2O indicate the optical density values of the calibrator and water at 412 nm, respectively, at 10 min. The term “n” denotes the dilution factor used in the analysis. Notably, the number “200” signifies the equivalent activity of the calibrator, as determined under the specific assay conditions.
Determination of epinephrine: The technique outlined by Kema et al.22 was utilized to assess the level of epinephrine. To begin, in the pre-prepared wells of the norepinephrine Microtiter Strips, 25 μL of the enzyme solution was dispensed. Following that, 20 μL of extracted standards, controls and samples were added to their respective wells. Subsequently, the mixtures were incubated for 30 min at room temperature (20-25°C) on a shaker set, operating at approximately 600 rpm.
Following this, 50 μL of nor-epinephrine antiserum was added to all the wells and the plate was sealed with an Adhesive Foil. The foils were later removed and the contents of the wells were discarded. The plates underwent three wash cycles with 300 μL of wash buffer each time, with the contents being discarded and the plate dried by tapping it on absorbent material while in an inverted position.
Subsequently, 100 μL of enzyme conjugate was introduced into the wells and they were subjected to another 30 min incubation at room temperature on the shaker (at roughly 600 revolutions per minute (rpm)). The liquids within the wells were aspirated once again and the plates underwent three additional wash cycles using wash buffer. After each wash, the content was discarded, then, the plate was dried by tapping it gently on absorbent material while in an inverted position.
To each well, 100 μL of substrate was introduced, following that, the plate underwent another 5 min incubation at 25°C while placed on the shaker set to operate at approximately 600 rpm. To conclude, 100 μL of stop solution was added into each well and the microtiter plate was agitated to ensure that there is even distribution in the solution.
The solutions’ absorbance in each well was read using a microplate reader set to 450 nm within a 10 min time frame. The concentrations of both the samples and controls were directly deduced using the standard curve.
Determination of nor-epinephrine: The level of nor-epinephrine was assessed using the technique of Kema et al22. In the pre-prepared wells of the nor-epinephrine Microtiter Strips, 25 μL of the enzyme solution was introduced. Subsequently, 20 μL of the extracted controls, standards and samples were introduced to their respective wells. The mixtures were subsequently incubated at room temperature (20-25°C) for thirty minutes on a shaker set to operate at approximately 600 rpm.
Following that, 50 μL of norepinephrine antiserum was introduced into each well and the plate was sealed with Adhesive Foil. The foils were subsequently removed and the contents of the wells were discarded. The plates underwent three wash cycles by adding 300 μL of Wash Buffer each time and after each wash, the contents were discarded and the plate was dried by tapping it on absorbent material while in an inverted position.
Following that, 100 μL of enzyme conjugate was introduced into the wells and the plate underwent another 30 min incubation at room temperature on the shaker set to operate at 600 rpm. The contents of the wells were aspirated once more and the plates were subjected to three additional wash cycles using Wash Buffer. After each wash, the content was discarded and the plate was dried by tapping it on absorbent material while in an inverted position.
To each well, 100 μL of substrate was introduced and the plate was subsequently incubated at room temperature for 25 min on the shaker (approximately 600 rpm). Finally, 100 μL of stop solution was introduced into all the wells and the plate was agitated so that the constituents of each solution mix properly.
The microplate reader measured the absorbance of the solution in the wells within a 10 min timeframe at a wavelength of 450 nm. The concentrations of the samples and controls were directly derived from the standard curve.
Determination of Serotonin: The level of serotonin was determined following the procedure established by Kema et al.22. In the Serotonin/5-HIAA Microtiter Strips, 100 μL each of controls, acylated standards and samples were introduced into their wells. Afterwards, 25 μL of serotonin antiserum was added to these wells. The plate was sealed with Adhesive Foil and allowed to incubate for 15-20 hrs at a temperature range of 2-8°C.
Following the incubation period, the foil was taken off and the contents of the wells were discarded. The plate was subjected to three wash cycles by adding 300 μL of Wash Buffer and after each wash, the contents were discarded and the plate was dried by tapping it on absorbent material while in an inverted position.
Subsequently, 100 μL of enzyme conjugate was introduced into the wells and allowed to incubate for 30 min at 25°C on a shaker (approximately 600 rpm). After the incubation, the substances within the wells were disposed of and the plate underwent three additional wash cycles using Wash Buffer. After each wash, the content was discarded and the plate was dried by tapping it on absorbent material while in an inverted position.
To each well, 100 μL of substrate was introduced and the plate was subsequently incubated for approximately 30 min at 25°C using the shaker set to operate at 600 rpm. Finally, 100 μL of stop solution was added equally to each well and the microtiter plate was agitated for proper mixing of the solution.
The absorbance of the solutions in the wells was measured within a 10 min timeframe using a microplate reader set to 450 nm. The concentrations of both the samples and controls were directly derived from the standard curve.
Determination of dopamine: The method described by Kema et al.22 was employed to ascertain the dopamine level. For each pre-prepared well in the Dopamine Microtiter Strips, 25 μL of the enzyme solution was introduced. Subsequently, 25 μL of extracted standards, 50 μL of extracted samples and 25 μL of controls and were added to their respective wells. Additionally, to each of the samples, controls and standards, 25 μL of hydrochloric acid was added. The content of the solutions were then incubated for 30 min at room temperature using a shaker set to operate at 600 rpm.
Afterwards, 50 μL of dopamine antiserum was introduced to the wells and the plate was sealed using Adhesive Foil. The mixtures were then left to incubate for an additional 2 hrs at room temperature on the shaker (approximately 600 rpm). Subsequently, the foils were taken off and the contents of the wells were either disposed of or aspirated.
The plate was washed three times by adding 300 μL of Wash Buffer and after each wash, the contents were discarded and the plate was dried by tapping it on absorbent material while inverted.
Afterwards, 100 μL of enzyme conjugate was introduced into the wells and the plate was incubated for an additional 30 min at 25°C on the shaker (approximately 600 rpm). Following this, the contents of the wells were either discarded or aspirated.
Subsequently, the plate was subjected to three additional washes using Wash Buffer. After each wash, the content was discarded and the plate was dried by gently tapping it on an absorbent material while inverted.
In each well, 100 μL of substrate was introduced and the plate was then incubated for 25±5 min at room temperature while placed on a shaker set to operate at 600 rpm. Finally, 100 μL of the stop solution was introduced into each well and the microtiter plate was agitated so that the contents of each solution mixes properly.
Statistical analysis: The data underwent analysis using one-way ANOVA with GraphPad Prism 8.0.2. To make group comparisons, a post hoc Dunnett’s Multiple Comparison Test was put into use. The results were displayed as the Mean±Standard Error of the means (SEM). Statistical significance was established when the p-value was below 0.05.
RESULTS
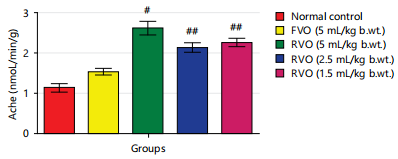
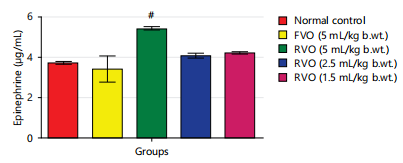
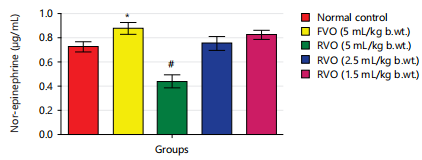
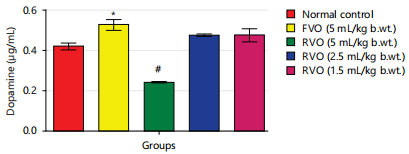
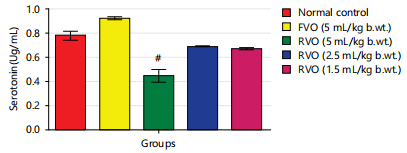
Effects of reused vegetable oil (RVO) on some brain neurotransmitters in normal albino rats: Dopamine, serotonin and nor-epinephrine levels were notably diminished (p<0.05) in rats that received RVO when compared to groups that received fresh vegetable oil and normal control (Fig. 1, 2, 3, 4 and 5). Significant (p<0.05) elevations were observed in epinephrine level and activity of AchE in rats that received RVO (Fig. 3).
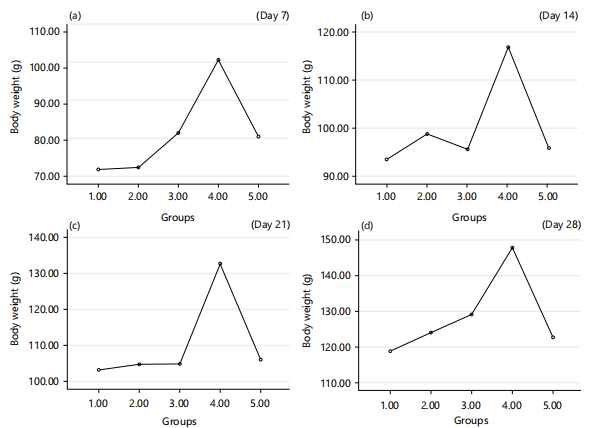
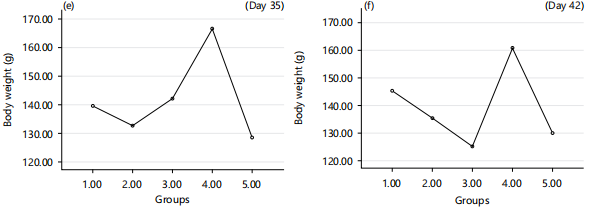
Effects of fresh and reused vegetable oil (RVO) on body weight of normal albino rats: There was a significant (p<0.05) increase in body weight of the albino rats that received RVO and FVO when compared to normal group in time dependent manner as shown in Fig. 6.
|
|
|
|
|
DISCUSSION
The process of deep-frying edible oils causes fats and oils to undergo oxidative degradation, leading to the release of free radicals. Free radical-initiated oxidation is a primary factor contributing to the rancidity of fats and oils, resulting in changes to essential quality attributes such as color, flavor, aroma and nutritional value23. The brain is especially susceptible to oxidative stress because of its elevated concentration of non-heme iron, which actively contributes to the production of free radicals24. In this study, it was observed that the groups consuming reused vegetable cooking oil from fast food vendors in Abakaliki Metropolis exhibited a significant (p<0.05) decrease in the concentration of monoamine neurotransmitters (nor-epinephrine dopamine and serotonin) and a notable (p<0.05) elevation in acetylcholine esterase activity and epinephrine levels in the brain (Fig. 1-5).
|
The elevation in 5-HT, NE and DA levels observed in various central nervous areas of albino rats could be attributed to Ca2+/calmodulin binding inhibition; this plays a pivotal role in the release of neurotransmitters. These findings differ from those of Bawazir25, who reported a significant elevation in 5-HT, NE and DA levels in different brain regions (cerebellum, hypothalamus, striatum, brainstem, cerebral cortex, as well as hippocampus) of male albino rats following the olive oil administration (7.5mg/kg b.wt.). An explanation for this discrepancy lies in the fact that the olive oil used in Bawazir's25 study was unlike the reused vegetable oil utilized in the present study. This result corresponds with the research carried out by Ezzeldin et al.26, where it was demonstrated that administering tramadol orally once a day for 28 days resulted in a notable (p<0.05) decline in the concentration of monoamine neurotransmitters (dopamine (DA), serotonin (5-HT) and nor-epinephrine (NE)) in Sprague-Dawley rats with streptozotocin-induced diabetes. It also aligns with the findings of El-Baky and Hafez27, who documented a notable increase (p<0.001) in monoamine oxidase (MAO) activity associated with the plasma, resulting in a decline in brain tissue proteins of serotonin (5-HT) and dopamine in albino rats treated with tramadol.
The reduction in neurotransmitters caused by tramadol could be linked to its capacity to impede their uptake28 and hinder reuptake, along with interactions involving the O-desmethyltramadol, metabolite of tramadol and monoamine neurotransmitter receptors. The increase in brain acetylcholinesterase (AChE) activity triggered by this drug could partly account for the cognitive and memory impairments seen in users of tramadol or morphine. As a result, leading to several issues, including decline in work productivity and difficulties in social integration and may even pose a risk of fatalities. Furthermore, the increased drug dosages did not lead to substantial changes in most of the evaluated factors, as indicated by the initial observations. This outcome is possibly attributed to the development of tolerance and dependence due to prolonged opioid use. In a separate study, Elwy and Tabl 29 observed a noteworthy rise (p<0.05) in the activity of AChE in the cerebral cortex of the brain after administering therapeutic doses of either morphine or tramadol in distinct groups. The AChE is the enzyme accountable for facilitating the breakdown of the acetylcholine (ACh), yielding acetic acid and choline and it exhibits a higher rate of action on ACh when compared to other choline esters. Furthermore, normal AChE activity in the brain is vital for its healthy functioning and alteration in the activity of AChE are linked to noticeable signs of neurobehavioral toxicity. The cholinergic neurotransmission in the central nervous system is of utmost importance for cognitive activities such as learning and memory30. Therefore, alterations in central cholinergic activity can significantly impact cognitive functions.
The findings concerning body weight demonstrated a noticeable (p<0.05) increase in the body weight of albino rats that received RVO and FVO when compared with the normal group and this change was observed in a time-dependent manner, as depicted in Fig. 6. This result was in line with the report of Ahmad et al.31. This could be due to the accumulation fat on the body tissues due to the consumption of reused vegetable oil.
CONCLUSION
The findings of this study indicate that the consumption of reused or heated vegetable cooking oil from fast food vendors in Abakaliki poses a risk to the CNS and may elevate the likelihood of developing Alzheimer’s and Parkinson’s diseases. The administration of reused vegetable oil caused a notable (p<0.05) decrease in the concentration of dopamine and serotonin, as well as the level of nor-epinephrine and increased the activity of acetylcholinesterase and level of epinephrine in the brain of normal rats.
SIGNIFICANCE STATEMENT
This study highlights the detrimental effects of reused vegetable oil (RVO) on brain neurotransmitters in albino rats, emphasizing the significance of monitoring cooking oil quality in fast food establishments. Key findings reveal significant reductions in nor-epinephrine, dopamine and serotonin levels, alongside increased epinephrine levels and acetylcholinesterase activity in RVO-exposed rats. These results underscore the potential risks associated with RVO consumption, warranting further investigation into the broader implications for human health. Future research could explore interventions to mitigate oxidative stress induced by RVO consumption and assess long-term neurological consequences in human populations. Overall, this study underscores the importance of maintaining cooking oil quality standards to safeguard public health.
ACKNOWLEDGMENT
The authors are grateful for the efforts of the research assistants from the laboratory in which the work was performed.
REFERENCES
- Chakraborty, R., K. Bose and S.J. Ulijaszek, 2009. Income level and food intake patterns among male Bengalee slum dwellers in Kolkata, India. Malays. J. Nutr., 15: 19-25.
- Dhruv, S., S. Patel and U. Iyer, 2011. Snacking pattern of residents of vadodara: A pilot study. Int. J. Appl. Biol. Pharm. Technol., 2: 81-87.
- Leong, X.F., M.N.M. Najib, S. Das, M.R. Mustafa and K. Jaarin, 2009. Intake of repeatedly heated palm oil causes elevation in blood pressure with impaired vasorelaxation in rats. Tohoku J. Exp. Med., 219: 71-78.
- Gao, Y.J. and R.M.K.W. Lee, 2001. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br. J. Pharmacol., 134: 1639-1646.
- Theodora, P., N. Androniki, O. Philippos, T. Dimitrios, M. Theodoros and T. Antonia, 2004. Olive oil, the Mediterranean diet and arterial blood pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study1-3. Am. J. Clin. Nutr., 80: 1012-1018.
- Hashempour-Baltork, F., M. Torbati, S. Azadmard-Damirchi and G.P. Savage, 2016. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol., 57: 52-58.
- Mozaffarian, D., M.B. Katan, A. Ascherio, M.J. Stampfer and W.C. Willett, 2006. Trans fatty acids and cardiovascular disease. N. Eng. J. Med., 354: 1601-1613.
- Ng, C.Y., X.F. Leong, N. Masbah, S.K. Adam, Y. Kamisah and K. Jaarin, 2014. Reprint of “heated vegetable oils and cardiovascular disease risk factors”. Vasc. Pharmacol., 62: 38-46.
- Esther, L.G., M.B. Schulze, J.B. Meigs, J.E. Manson and N. Rifai et al., 2005. Consumption of Trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr., 135: 562-566.
- Saljoughian, S., S. Roohinejad, A.E.D.A. Bekhit, R. Greiner, A. Omidizadeh, N. Nikmaram and A.M. Khaneghah, 2018. The effects of food essential oils on cardiovascular diseases: A review. Crit. Rev. Food Sci. Nutr., 58: 1688-1705.
- Soriguer, F., G. Rojo-Martínez, M.C. Dobarganes, J.M. García Almeida and E. Isabel et al., 2003. Hypertension is related to the degradation of dietary frying oils. Am. J. Clin. Nutr., 78: 1092-1097.
- Adam, S.K., S. Das, I.N. Soelaiman, N.A. Umar and K. Jaarin, 2008. Consumption of repeatedly heated soy oil increases the serum parameters related to atherosclerosis in ovariectomized rats. Tohoku J. Exp. Med., 215: 219-226.
- Garrido-Polonio, C., M.C. García-Linares, M.T. García-Arias, S. López-Varela, M.C. García-Fernández, A.H.M. Terpstra and F.J. Sánchez-Muniz, 2004. Thermally oxidised sunflower-seed oil increases liver and serum peroxidation and modifies lipoprotein composition in rats. Br. J. Nutr., 92: 257-265.
- Adam, S.K., I.N. Soelaiman, N.A. Umar, N. Mokhtar, N. Mohamed and K. Jaarin, 2008. Effects of repeatedly heated palm oil on serum lipid profile, lipid peroxidation and homocysteine levels in a post-menopausal rat model. Mcgill J. Med., 11: 145-151.
- Rani, A.K.S., S.Y. Reddy and R. Chetana, 2010. Quality changes in trans and trans free fats/oils and products during frying. Eur. Food Res. Technol., 230: 803-811.
- Choe, E. and D.B. Min, 2007. Chemistry of deep-fat frying oils. J. Food Sci., 72: R77-R86.
- Choe, E. and D.B. Min, 2006. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr., 46: 1-22.
- Kamisah, Y., A. Adam, W.Z.W. Ngah, M.T. Gapor, O. Azizah and A. Marzuki, 2005. Chronic intake of red palm olein and palm olein produce beneficial effects on plasma lipid profile in rats. Pak. J. Nutr., 4: 89-96.
- Clemente, T.E. and E.B. Cahoon, 2009. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol., 151: 1030-1040.
- Seppanen, C.M., Q. Song and A.S. Csallany, 2010. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats and food systems. J. Am. Oil Chem. Soc., 87: 469-481.
- Ellman, G.L., K.D. Courtney, V. Andres Jr. and R.M. Featherstone, 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-90.
- Kema, I.P., A.M.J. Schellings, C.J.M. Hoppenbrouwers, H.M. Rutgers, E.G.E. de Vries and F.A.J. Muskiet, 1993. High performance liquid chromatographic profiling of tryptophan and related indoles in body fluids and tissues of carcinoid patients. Clin. Chim. Acta, 221: 143-158.
- Donnelly, J.K. and D.S. Robinson, 1995. Invited review free radicals in foods. Free Radical Res., 22: 147-176.
- Yousuf, S., S. Salim, M. Ahmad, A.S. Ahmed, M.A. Ansari and F. Islam, 2005. Protective effect of Khamira Abresham Uood Mastagiwala against free radical induced damage in focal cerebral ischemia. J. Ethnopharmacol., 99: 179-184.
- Bawazir, A.E., 2011. Chronic effect of olive oil on some neurotransmitter contents in different brain regions and physiological, histological structure of liver and kidney of male albino rats. World J. Neurosci., 1: 31-37.
- Ezzeldin, E., W.A.H. Souror, T. El-Nahhas, A.N.M.M. Soudi and A.A. Shahat, 2014. Biochemical and neurotransmitters changes associated with tramadol in streptozotocin-induced diabetes in rats. BioMed Res. Int., 2014.
- El Baky, A.E.A. and M.M. Hafez, 2017. NOS expression in oxidative stress, neurodegeneration and male infertility induced by the abuse of tramadol. Biochem. Pharmacol., 6.
- Halfenny, D.M., L.F. Callado, S.E. Hopwood, T.A. Bamigbade, R.M. Langford and J.A. Stamford, 1999. Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Br. J. Anaesth., 83: 909-915.
- Elwy, A.E.H.M. and G. Tabl, 2017. Impact of tramadol and morphine abuse on the activities of acetylcholine esterase, Na+/K+-ATPase and related parameters in cerebral cortices of male adult rats. Electron. Physician, 9: 4027-4034.
- Schliebs, R. and T. Arendt, 2011. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res., 221: 555-563.
- Ahmad, N., S. Majumder, M.A. Miah and M.J. Uddin, 2007. Effects of edible fats and oils on the body weight gain and on weights of some selected organs in rats removing the impact of unequal feed intake. Bangladesh J. Vet. Med., 5: 107-110.
How to Cite this paper?
APA-7 Style
Asouzu,
N.C., Eze,
E.D., Aja,
P.M., Moyosore,
A.A., Chinaza,
A.G., Lucy,
A., Ben,
O.M., Asouzu,
N.C., Ifunanya,
O.A., Atoki,
A.V. (2024). Effects of Reused Vegetable Cooking Oil by Fast Food Vendors in Abakaliki Metropolis on Brain Neurotransmitters in Normal Albino Rats. Asian Journal of Biological Sciences, 17(4), 599-609. https://doi.org/10.3923/ajbs.2024.599.609
ACS Style
Asouzu,
N.C.; Eze,
E.D.; Aja,
P.M.; Moyosore,
A.A.; Chinaza,
A.G.; Lucy,
A.; Ben,
O.M.; Asouzu,
N.C.; Ifunanya,
O.A.; Atoki,
A.V. Effects of Reused Vegetable Cooking Oil by Fast Food Vendors in Abakaliki Metropolis on Brain Neurotransmitters in Normal Albino Rats. Asian J. Biol. Sci 2024, 17, 599-609. https://doi.org/10.3923/ajbs.2024.599.609
AMA Style
Asouzu
NC, Eze
ED, Aja
PM, Moyosore
AA, Chinaza
AG, Lucy
A, Ben
OM, Asouzu
NC, Ifunanya
OA, Atoki
AV. Effects of Reused Vegetable Cooking Oil by Fast Food Vendors in Abakaliki Metropolis on Brain Neurotransmitters in Normal Albino Rats. Asian Journal of Biological Sciences. 2024; 17(4): 599-609. https://doi.org/10.3923/ajbs.2024.599.609
Chicago/Turabian Style
Asouzu, Nwabunma, C., Ejike Daniel Eze, Patrick Maduabuchi Aja, Afodun Adam Moyosore, Awuchi Godswill Chinaza, Aja Lucy, Okon Michael Ben, Nonso C. Asouzu, Okide Afoma Ifunanya, and Ayomide Victor Atoki.
2024. "Effects of Reused Vegetable Cooking Oil by Fast Food Vendors in Abakaliki Metropolis on Brain Neurotransmitters in Normal Albino Rats" Asian Journal of Biological Sciences 17, no. 4: 599-609. https://doi.org/10.3923/ajbs.2024.599.609

This work is licensed under a Creative Commons Attribution 4.0 International License.



