Black Stem of Sunflower: An Emerging Fungal Disease Caused by Fusarium solani in Bangladesh
| Received 06 Jul, 2024 |
Accepted 09 Sep, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Sunflower is one of the world's most important oil seed crops. However, these crops are prone to biotic factors, which reduce the yield of the crops. The present experiment was designed to identify and assess the growth characteristics of the fungal pathogens associated with the black stem disease of sunflowers and its control measures in Bangladesh. Materials and Methods: Both morphological features and molecular characterization based on the rDNA ITS region of the fungus were employed to identify the fungal pathogen. Six distinct solid fungal culture media, five separate temperature ranges and five different pH levels of medium were used to assess the mycelial growth behavior of the isolated fungus. Selected biocontrol agents and chemical fungicides were employed against the fungus under in vitro conditions. Results: The BLASTn search on the NCBI database revealed that the fungal pathogen (accession number: PP573854.1) showed above 99% homology with previously deposited sequences of Fusarium solani. Koch’s postulate test was conducted to prove the pathogenicity. The fungal culture media, Potato Sucrose Agar and Carrot Agar exhibited the maximum mycelial growth. The vegetative growth of the fungus was most favored at 30°C temperature. Besides, slightly acidic conditions were mostly preferred by the F. solani. Among the fungal antagonists, Trichoderma erinaceum inhibited the maximum (81%) of the mycelial growth of the targeted fungus, followed by 73% for Trichoderma harzianum. Fungicides Amistar Top 325 SC and copper blue 50 WG showed above 70% growth inhibition against the vegetative growth under in vitro conditions. Conclusion: Biocontrol agents and synthetic fungicides will be promising options to control the black stem diseases of sunflowers. Black stem disease on sunflowers caused by Fusarium solani is a new record in Bangladesh.
| Copyright © 2024 Sikder et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The sunflower (Helianthus annum L.) is a flowering plant from the Asteraceae family. It is native to the North American Region1. It is one of the main vegetable oil sources globally, ranking after rapeseed, palm and soybean2. It is an important oilseed crop, contains a good percentage of oil (48-53), protein (14-19), crude fiber (16-27), ash (2-3), soluble sugar (7-9) and hull (21-27). Its short duration andphoto-insensitivity suit it well for cultivation in the rainy season3. The sunflower is cultivated globally as an oil seed crop which yields one of the world’s most important sources of cholesterol-free, unsaturated edible oil with high vitamin E content. For this reason, the popularity of this oil is increasing day by day worldwide.
Various biotic factors are responsible for the economic losses of sunflowers. Several fungal pathogens cause major diseases of the crop. Fusarium spp. was previously considered a minor pathogen in sunflowers4. However, Fusarium spp., F. oxysporum var. orthoceras; F. sporotrichioides, was found to be serious pathogens in sunflowers in different states of the USA, Argentina and Russia where damage up to 80% has been reported by Gulya et al.5 and Leslie and Summerell6. Besides, a survey was undertaken in the U.S. (Minnesota, North Dakota and South Dakota) and Argentina in 2010 to determine stalk rot pathogens of sunflowers; aggressiveness of the eight species of Fusarium, in which F. equiseti and F. sporotrichioides were the most aggressive species, followed by F. acuminatum7,8. In Bangladesh, Sikder et al.9 reported sunflower stem rot diseases and are responsible for lowering seed production in Bangladesh. However, researchers in our country have not conducted a comprehensive study on sunflower black stem disease. The present study was conducted to detect the black stem-causing fungal pathogen of sunflower plants via morphological and molecular approaches; to assess the effect of fungal culture media and environmental factors on the vegetative growth of the isolated fungus; to evaluate the impact of bio-control agents and fungicides against the targeted fungus.
MATERIALS AND METHODS
Sample collection and isolation of fungal pathogen: At the beginning of the research work, several farmer’s fields located in Mankigonj District, Bangladesh were visited to visually observe disease incidence during July and August, of 2021. After the rainy seasons, blackish symptoms appeared on the stem above the base of the sunflower plants. At least 10 diseased samples were collected in sterilized zipper bags from the selected fields. Those samples were washed in running tap water several times and dried on sterile Petri dishes. Next, samples were chopped into small pieces, followed by surface sterilization using 5% sodium hypochlorite for 5 min. Then, samples were washed several times with sterile water to remove disinfectants. Afterward, the samples were air-dried, placed on potato dextrose agar (PDA) medium and incubated at room temperature (25±2°C) for 7 days. The hyphal tip method was used to isolate purified fungal pathogens.
Identification of pathogen: Firstly, morphological features such as colony characters, pigmentation, hyphal septation and branching pattern, conidia and conidiophore structure, chlamydospores formation pattern, etc. were investigated. Besides, the genomic DNA of the isolated fungus was extracted according to the procedure described by Sultana et al.10. Polymerase Chain Reaction (PCR) was performed to amplify the internal transcribed spacer (ITS) region of the fungus using specific primer sets ITS5 (5-GGAAGTAAAAGTCGTAACAAGG-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) as the method described by White et al.11. To amplify the fungal ITS sections, a PCR reaction was conducted in a 25 μL volume having 1 μL of a DNA template, 12.5 μL of a reaction mixture containing dNTPs, Buffer, MgCl2, Taq Polymerase (Origin: Promega, USA), 1 μL each of forward and reverse primers (20 pMol) and 9.5 μL H2O. The PCR cycles consist of an initial denaturation at 95°C for 3 min, 35X (95°C for 30 sec, 48°C for 30 sec and 72°C for 50 sec), termination at 72°C for 5 min and overnight hold at 4°C9,12. The PCR product was run on agarose gel, then purified using PCR Clean-Up System (Promega, USA) and sent to FIRST BASE Laboratories (Sdn Bhd, Kuala Lumpur, Malaysia) for sequencing. The neighbor-joining tree was generated using the MEGA 11 version 11.0.13 software.
Koch’s postulates for pathogenicity: To fulfill Koch’s postulates, an in vivo experiment was carried out in the experimental site of the Botanical Garden, Department of Botany, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh. The healthy seedlings were grown in potting media. After 45 days, the stems of five plants were injured using a sterile blade and the mycelial plug was inoculated on the stem and carefully wrapped with parafilm (Fig. 1). The leaf blades of five sunflower plants were removed using a sterile blade and the petiole was placed in a fungal inoculum-loaded eppendorf tube (200 μL). The plants treated without inoculum served as a control. Those plants were kept under controlled environmental conditions for 2 weeks. After initiating the disease symptom, re-isolation of the fungal pathogen was done to prove the pathogenic nature of a particular fungus.
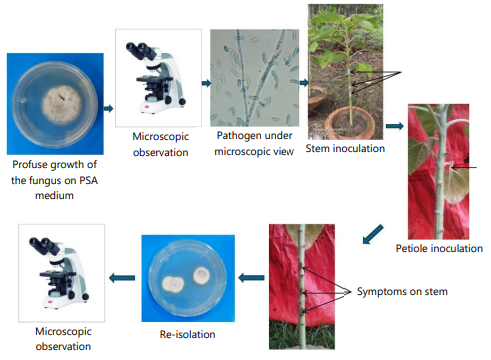
|
Evaluation of the effects of culture media, temperature and pH on fungal growth: Culture media have different nutrient compositions that influence the metabolism of the fungus. The impact of six unlike fungal culture media viz., potato sucrose agar (PSA), carrot agar (CA), sabouraud dextrose agar (SDA), yeast extract agar (YEA), honey peptone agar (HPA) and hansen’s agar (HA) on the mycelial growth of the fungus were assessed as mentioned by Sikder et al.13.
Temperature is known to stimulate fungal growth and proliferation. The role of five different temperature conditions viz., 15, 20, 25, 30 and 35°C on the mycelial growth of the identified fungus was assessed as described by Ahmmed et al.14.
Substrate pH also determines the fungus's growth and development. The effect of five different pH levels (5.0, 6.0, 7.0, 8.0 and 9.0) on the fungus's radial growth characteristics was determined as mentioned by Sultana et al.15.
Efficacy of antagonistic fungi and fungicides against the tested fungus: For an eco-friendly disease management system, antagonistic microorganisms are the best tools. The isolated fungus was tested against three Trichoderma spp., viz., T. harzianum (accession number: MH368147.1), T. erinaceum (lab collection) and T. asperellum (accession number: MH368120.1)16. A 2 mm diameter agar disc containing tested fungus of 7 day old cultures and 7 day culture of Trichoderma isolates were placed an equal distance in the Petri plate containing PDA. Three replications were maintained during the study. Only the centrally placed tested fungal disc was inoculated on the control plate. The inoculated plates were sealed with parafilm and incubated at 25±2°C temperatures for 7 days. The radial mycelial growth was measured after 7 days of incubations. The radial mycelial growth of the experimented plates was calculated and compared with the control plate.
The growth inhibition percentage of the tested fungus was determined using the following formula13:
Where:
| I | = | Mycelium growth inhibition (%) | |
| C | = | Vegetative growth in the control plate | |
| T | = | Vegetative growth in the treatment plate |
The effect of three different fungicides, namely Mycoguard 80 WP (mancozeb+metalaxyl), copper blue 50 WG (copper oxychloride) and Amistar Top 325 SC (azoxystrobin+difenoconazole) on the mycelial growth of isolated fungus using the food poison technique17. The requisite amounts of fungicides were mixed into a PSA medium with a concentration of Mycoguard at 1000, 1500 and 2000 ppm; copper blue at 2000, 2500 and 3000 ppm and Amistar Top at 1000, 1500 and 2000 ppm. Fungicides were mixed with the media after autoclaving. Six replicates of PSA plates were used for every dose of the respective fungicide. The PSA plate that received no fungicide served as control. Each Petri plate was inoculated with the mycelial disc of the test fungal pathogen and was taken from the 7-day-old colony on PSA. The inoculated plates were incubated at 25±2°C. The colony diameter was recorded after 7 days post-inoculation (dpi). The mycelial growth inhibition percentage was calculated using the previously described formula.
Statistical analysis: Data was analyzed using the statistical software SPSS-16 at a 5% level of significance. The phylogenetic tree was generated using MEGA 11 version 11.0.13 software.
RESULTS
Identification and pathogenicity of the fungus: The black discoloration appeared on the developing sunflower stem and extended to the petiole (Fig. 2a). The fungal colony was circular depending on culture media, with abundant white-colored, cottony/wooly appearance and elevated conidial masses with smooth margins (Fig. 2b). The mycelia were septate, hyaline and abundantly branched under microscopy (Fig. 2c). Chlamydospore formation was found (Fig. 2d). The microconidia evolved on separate, hyaline conidiophores that were well-developed. Microconidia were hyaline, oval-ellipsoidal shaped and aseptate (Fig. 2e), while macroconidia and acervuli were not found.
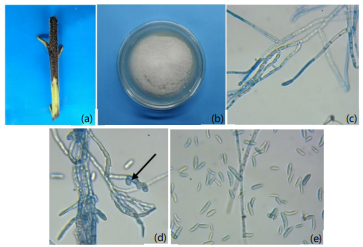
|
|
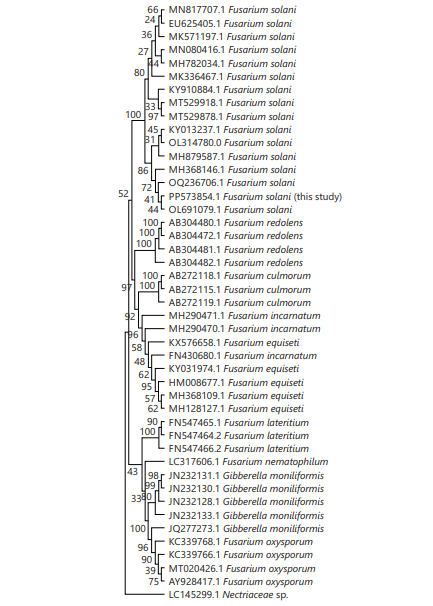
In the BLAST search, the ITS sequence of our fungal pathogen (PP573854.1) showed above 99% similarity with F. solani, which suggested the taxonomic position of the studied fungus. In the neighbor-joining tree, there were six distinct clusters of the different species of Fusarium, where the currently studied fungal pathogen F. solani (PP573854.1) was grouped into a separate cluster with F. solani clade with a bootstrap value of 100 (Fig. 3).
|
|
Typical black stem symptoms were observed on the stem; however, no symptoms were not found on the petiole. The pathogenic nature of F. solani with black stem disease of sunflower was confirmed through the pathogenicity test.
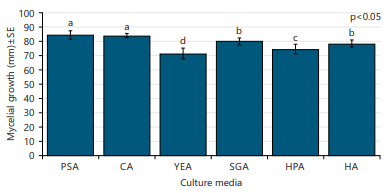
Fungal biology of the studied organism: The media components are important criteria for fungal culture and study and important physiological parameters that lead to maximum sporulation in fungi. The effect of six different solid media viz., potato sucrose agar (PSA), carrot agar (CA), yeast extract agar (YEA), sabouraud dextrose agar (SGA), honey peptone agar (HPA) and hansen’s agar (HA) on mycelial growth of F. solani was evaluated (Fig. 4). The results showed that there is a significant effect of culture media on the mycelial growth of F. solani (p<0.05) and the highest mycelial growth (84.25 mm) was obtained on PSA medium which was statistically similar to CA medium (83.78 mm), followed by SGA (79.78 mm) and HA (78.22 mm) media and the lowest mycelial growth (71.22 mm) on YEA medium (Fig. 4). The colony was whitish, cottony, elevated and smooth margin on PSA, whitish, cottony, emerged and smooth margin on CA, whitish, woolly, emerged and smooth margin on YEA, SGA, HPA, off-white, cottony, emerged and smooth margin on HA medium.
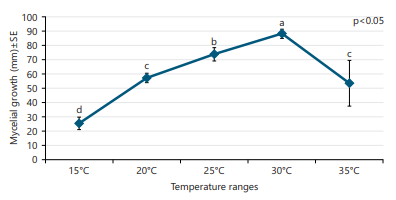
The impact of different temperature conditions assessed on the vegetative growth of F. solani on PSA media under in vitro conditions (Fig. 5). The colony appearance was changed with the increasing temperatures, such as light yellow at 15°C, whitish grey at 20°C, whitish on the top side while brown at the bottom side at 30°C and whitish at 35°C. Data revealed a significant difference in response due to temperature conditions (p<0.05). There was an upward trend of vegetative growth of F. solani up to 30 and afterward started to decline. The maximum mycelial growth (88.22 mm) was obtained at 30°C, followed by 25°C (73.92 mm) and 20°C (57.12 mm) while the lowest growth (25.44 mm) was found at the extreme lowest temperature (15°C).
|
|
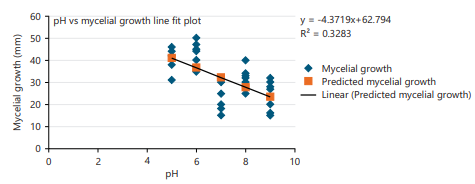
The experimental plates were incubated at five different pH levels viz., 5, 6, 7, 8 and 9 to find suitable pH conditions for the fungus. Data revealed that F. solani showed the maximum mycelial growth at acidic to neutral conditions (Fig. 6). The growth rate was comparatively slower at pH 8-9. However, pH conditions explained only 32% variation in the mycelial growth of the studied fungus.
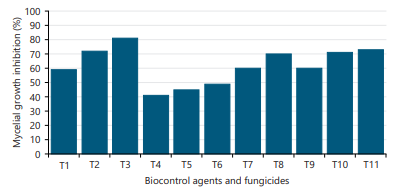
Role of antagonistic agents and fungicides to the fungus: Bio-control agents (Trichoderma harzianum, T. erinaceum and T. asperellum) showed promising results against the fungus. Among the three antagonists, T. erinaceum showed the maximum mycelial growth inhibition (81%) of F. solani followed by T. harzianum (73%) and T. asperellum (59%) (Fig. 7). The present study was also aimed to screen out the most effective chemical fungicides viz., Mycoguard 80 WG (mancozeb+metalaxyl), copper blue 50 WG (copper oxychloride) and Amistar Top 325 SC (azoxystrobin+difenoconazole) in low concentration against F. solani which cause black stem disease of sunflower. The result revealed that all three fungicides are effective against the fungus at moderately high concentrations in the current study. The growth inhibition percentage was highest (73%) at 2000 ppm concentration of Amistar Top, followed by 71 and 60% due to 1500 and 1000 ppm, respectively. Coper blue performed best (70%) at 3000 ppm concentration, followed by 68% for 2500 ppm and 60% for 2000 ppm concentration. Almost 50% growth inhibition was obtained at the 2000 ppm concentration of Mycoguard, followed by 1500 and 1000 ppm (Fig. 7).
DISCUSSION
Based on morphological characteristics, it was identified as Fusarium sp. Identifying the fungus at species rank without molecular characterization was difficult. Molecular techniques were used to determine the species rank. A BLASTN search showed that the fungus could be Fusarium solani. After generating the phylogenic tree, we confirmed the fungus’s taxonomic position. Sultana et al.18 also used similar techniques to identify the Fusarium at species rank.
In the current experiment, F. solani showed the highest mycelial growth in PSA and CA medium, which is supported by Mallik et al.19, who noted the 75.8 and 74.0 mm mycelial growth of F. solani in PSA and CA media, respectively. Osono and Takeda20 stated that reduced glucose content in fungal culture media inhibits the expansion of quickly developing species and promotes sporulation, which facilitate the identification of various fungal species. Observable morphological characteristics remain the basic foundation of fungal systematics. Thus, the phenotypes of fungi are used to identify and recognize them. Likewise, Lazarotto et al.21 also recorded the utmost vegetative growth and sporulation of Fusarium chlamydosporum in the PSA medium. Sultana et al.18 found the highest vegetative growth of Fusarium fujikuroi species complex on honey peptone agar, PSA and PDA media. Another bioassay revealed that the PDA and CA culture media were appropriate for Fusarium equiseti causing wilt disease of Centella asiatica22. Therefore, PSA, CA and PDA media are most suitable for the vegetative growth of F. solani.
Current study results revealed that the 30°C temperature was mainly appropriate for the vegetative growth of F. solani. Previous bioassays also showed that the temperature range between 25 to 30°C was most suitable for growth and sporulation of F. solani, F. tricinctum and F. oxysporum23-25. Akter et al.22 reported the maximum mycelial growth of F. equiseti at 25°C temperature. Another study concluded that a temperature of 22°C or above was reported as an optimum for F. oxysporum26. Hence, investigation suggests that 30°C is most suitable for the proliferation of the mycelium of F. solani.
In the present study, F. solani grew better at slightly acidic conditions, which conforms with the findings of Gordon et al.27 reported that the frequency of root infection of the strawberry cultivar Albion due to Fusarium oxysporum f. sp. fragariae, was significantly higher for plants grown in acidified soil (near pH 5) than soil near neutrality. Likewise, Groenewald et al.28 noted the most suitable growth of different isolates of Fusarium oxysporum f. sp. cubense at a pH level of 5-6. Another lab bioassay29 showed the optimum growth and survival of Fusarium oxysporum f. sp. elaeidis was found between 5.0 and 7.0. In other lab studies, neutral to slightly alkaline conditions were most suitable for the vegetative growth of Fusarium oxysporum30-32. Current results suggested that an increased pH level of the substrate could limit the growth of the pathogen at an economic level; however, more studies are needed to confirm this.
The most promising results were obtained when biocontrol agents were used to restrict the vegetative growth of the fungus. Around 80 and 70% of mycelial growth inhibition of the targeted fungus were found by T. erinaceum and T. harzianum, respectively. Previous results suggest that T. erinaceum bio-priming modulated the defense mechanism in plants along with the accumulation of higher antioxidative enzyme activities after challenging through F. oxysporum33. Besides, Herath et al.34 assessed the antagonistic activity of T. erinaceum against plant pathogenic fungi and found promising results. Biocontrol agent T. erinaceum was found to be an effective fungus in controlling several soil-borne plant pathogens (i.e., Rhizoctonia solani, Sclerotium rolfsii and Sclerotium oryzae) both under in vitro and in vivo conditions35. Rojo et al.36 reported on the biological control of F. solani causing peanut brown root rot disease by Trichoderma sp. and found that T. harzianum is an effective biological control agent. Lian et al.37 reported that T. harzianum conidia has been proven to restrict the growth of F. oxysporum, reduce disease progression and enhance crop productivity. Several reports suggested that T. harzianum was an effective antagonist against soil-borne fungal pathogens38-40. Furthermore, a previous study indicated that T. asperellum was shown to be an effective biocontrol agent against Fusarium oxysporum f. sp. niveum41. Hence, biocontrol agents could be used to restrict the vegetative growth of F. solani.
Both chemical fungicides, viz., Amistar Top 325 SC (azoxystrobin+difenoconazole) and copper blue (copper oxychloride), showed a significant reduction in the vegetative growth of the fungus. In Egypt, Al-Kahal et al.42 reported that copper oxychloride was the most effective against F. solani causing root rot disease of faba bean. Mallik et al.19 reported that Amistar Top 325 SC showed more than 50% mycelial growth restriction of F. solani by 750 ppm concentration. Masoud et al.43 studied that the fungicide Azoxystrobin provides the best results against targeted fungi. The highest level of inhibition was found using the fungicide Azoxystrobin (Amistar 25% SC). Data revealed that Amistar Top 325 SC inhibited (88.9%) mycelia growth of F. solani. Hence, current study results suggest that Amistar Top and copper blue are effective fungicides that could be applied to control the mycelial growth of F. solani.
CONCLUSION
The black stem of sunflowers caused by F. solani is emerging as a significant fungal disease, which is becoming a serious threat to the cultivation of sunflowers in Bangladesh. This disease has the potential to significantly reduce sunflower yields, hampering regional attempts to improve oilseed production. Correct identification of the disease-causing organism was done using both molecular and morphological approaches. Pathogens preferred specific environmental conditions such as temperature of 30°C and pH 5-6. Integrated disease management practices, including the use of resistant varieties, early sowing, change of soil pH and judicious application of fungicides, incorporation of bio-pesticides are essential for controlling the pathogen. To provide long-lasting solutions and viability of sunflower crops in Bangladesh, more study and observation are required.
SIGNIFICANCE STATEMENT
Sunflower is one of the most edible oil-yielding crops. Farmers face heavy losses due to fungal infestations. Correctly identifying the fungus is an essential step in formulating management practices. In this study, both morphological and molecular techniques were applied to identify the fungal pathogen. The pathogenic nature of the identified fungus was also determined. Results revealed that 30°C temperature was an ideal condition for the mycelial growth of the fungus. Lab bioassay revealed that biocontrol agents, viz., Trichoderma erinaceum and Trichoderma harzianum and fungicides Amistar Top and copper blue, were effective against the isolated fungus. The results of this present study will help to formulate the effective management of black stem diseases and enhance the quality of seed production of sunflower plants.
ACKNOWLEDGMENT
We acknowledge Dr. Durga Das Bhowmik for his kind help during the field visits and samplings.
REFERENCES
- Yadava, D.K., S. Vasudev, N. Singh, T. Mohapatra and K.V. Prabhu, 2011. Breeding Major Oil Crops: Present Status and Future Research Needs. In: Technological Innovations in Major World Oil Crops, Volume 1: Breeding, Gupta, S.K. (Ed.), Springer, New York, ISBN 978-1-4614-0356-2, pp: 17-51.
- Fernández-Martínez, J.M., B. Pérez-Vich and L. Velasco, 2009. Sunflower. In: Oil Crops, Vollmann, J. and I. Rajcan (Eds.), Springer, New York, ISBN: 978-0-387-77594-4, pp: 155-232.
- Thimmegowda, M.N., H.V. Nanjapa and B.K. Ramachandrappa, 2007. Effect of soil solarization and farmyard manure application on weed control and productivity of sunflower (Helianthus annuus)-bell pepper (Capsicum annum) sequence. Indian J. Agron., 52: 204-207.
- Gulya, T., K.Y. Rashid and S.M. Masirevic, 1997. Sunflower Diseases. In: Sunflower Technology and Production, Schneiter, A.A. (Ed.), Soil Science Society of America, Inc., Madison, Wisconsin, ISBN: 9780891182276, pp: 263-379.
- Gulya, T.J., F. Mathew, R. Harveson, S. Markell and C. Block, 2018. Diseases of Sunflower. In: Handbook of Florists' Crops Diseases, McGovern, R.J. and W.H. Elmer (Eds.), Springer, Cham, ISBN: 978-3-319-39670-5, pp: 787-837.
- Leslie, J.F. and B.A. Summerell, 2006. The Fusarium Laboratory Manual. 1st Edn., Blackwell Publishing, Oxford, United Kingdom, ISBN: 9780813819198, Pages: 388.
- Mathew, F., B. Kirkeide, T. Gulya and S. Markell, 2010. First report of pathogenicity of Fusarium sporotrichioides and Fusarium acuminatum on sunflowers in the United States. Plant Health Prog., 11.
- Mathew, F.M., K.M. Alananbeh, J.G. Jordahl, S.M. Meyer, L.A. Castlebury, T.J. Gulya and S.G. Markell, 2015. Phomopsis stem canker: A reemerging threat to sunflower (Helianthus annuus) in the United States. Phytopathology, 105: 990-997.
- Sikder, M.M., M.S. Ahmmed, P. Akter, F.A. Shetu and B. Akhter et al., 2024. First report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) causing basal stem rot disease on sunflower in Bangladesh. Asian J. Biol. Sci., 17: 448-461.
- Sultana, S., M.M. Sikder, M.S. Ahm and N. Alam, 2022. Neopestalotiopsis chrysea causing leaf spot disease of strawberry plants in Bangladesh. J. Plant Sci., 17: 66-74.
- White, T.J., T. Bruns, S. Lee and J. Taylor, 1990. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: PCR Protocols: A Guide to Methods and Applications, Innis, M.A., D.H. Gelfand, J.J. Sninsky and T.J. White (Eds.), Academic Press, Elsevier Inc., ISBN: 9780123721808, pp: 315-322.
- Ahmmed, M.S., M.M. Sikder, S.R. Sarker, M.S. Hossain, R.D. Shubhra and N. Alam, 2022. First report of Pseudopestalotiopsis theae causing Aloe vera leaf spot disease in Bangladesh. Am. J. Plant Sci., 13: 193-204.
- Sikder, M.M., M.S. Ahmmed, A. Sultana and N. Alam, 2021. First report on black spot disease of Phyllanthus emblica L. fruits caused by Thielaviopsis paradoxa in Bangladesh. Int. J. Agric. Res. Innovation Technol., 10: 38-46.
- Ahmmed, M.S., M.M. Sikder and N. Alam, 2022. Molecular characterization of endophytic fungi-Daldinia eschscholtzii from Aloe vera plants in Bangladesh. Jahangirnagar Univ. J. Biol. Sci., 11: 1-11.
- Sultana, A., M.M. Sikder and N. Alam, 2023. Identification and fungal biology of post-harvest fungi associated with amla fruits and it's in vitro control measures. Adv. Zool. Bot., 11: 1-11.
- Ahmmed, M.S., M.M. Sikder, A. Sultana and N. Alam, 2022. First report of leaf spot disease of Aloe vera caused by Nigrospora sphaerica in Bangladesh. J. Plant Sci., 17: 95-101.
- Billah, M.B., M.M. Sikder, M.R.I. Mallik, M.K. Hossain and N. Alam, 2021. Molecular characterization and vegetative growth of pathogenic seed-borne fungus, Curvularia lunata of tomato and its in vitro control measures. Int. J. Agric. Res. Innov. Tech., 11: 124-132.
- Sultana, S., M.M. Sikder, F.A. Neela and N. Alam, 2023. First report of strawberry leaf blight caused by Fusarium fujikuroi species complex in Bangladesh. J. Bio-Sci., 30: 11-20.
- Mallik, M.R.I., M.M. Sikder, M.K. Hossain, M.B. Billah and N. Alam, 2022. Molecular characterization and in vitro control measures of fruit rot disease of sweet pepper. Int. J. Agric. Res. Innov. Tech., 11: 108-116.
- Osono, T. and H. Takeda, 1999. A methodological survey on incubation of fungi on leaf litter of Fagus crenata. Appl. For. Sci., 8: 103-108.
- Lazarotto, M., R. Mezzomo, C.G. Maciel, G. Finger and M.F.B. Muniz, 2014. Mycelia growth and sporulation of Fusarium chlamydosporum species complex under different culture conditions. Amazonian J. Agric. Environ. Sci., 57: 35-40.
- Akter, P., S. Khatun, D.D. Bhowmik, F.A. Neela and N. Alam, 2022. Vegetative growth and molecular identification of Fusarium equiseti isolated from wilt disease of Centella asiatica L. in Bangladesh. Am. J. Plant Sci., 13: 294-305.
- Gupta, V., A. Misra and R. Gaur, 2010. Growth characteristics of Fusarium spp. causing wilt disease in Psidium guajava L. in India. J. Plant Protect. Res., 50: 452-462.
- Yan, H. and B. Nelson Jr., 2020. Effect of temperature on Fusarium solani and F. tricinctum growth and disease development in soybean. Canadian Journal of Plant Pathology 42: 527-537.
- Cruz, D.R., L.F.S. Leandro and G.P. Munkvold, 2019. Effects of temperature and pH on Fusarium oxysporum and soybean seedling disease. Plant Dis., 103: 3234-3243.
- Kaur, S., R. Barakat, J. Kaur and L. Epstein, 2022. The effect of temperature on disease severity and growth of Fusarium oxysporum f. sp. apii races 2 and 4 in Celery. Phytopathology, 112: 364-372.
- Gordon, T.R., M. Stueven, A.M. Pastrana, P.M. Henry, C.M. Dennehy, S.C. Kirkpatrick and O. Daugovish, 2019. The effect of pH on spore germination, growth, and infection of strawberry roots by Fusarium oxysporum f. sp. fragariae, cause of Fusarium wilt of strawberry. Plant Dis., 103: 697-704.
- Groenewald, S., N. van den Berg, W.F.O. Marasas and A. Viljoen, 2006. Biological, physiological and pathogenic variation in a genetically homogenous population of Fusarium oxysporum f.sp. cubense. Australas. Plant Pathol., 35: 401-409.
- Oritsejafor, J.J., 1986. Influence of moisture and pH on growth and survival of Fusarium oxysporum f.sp. elaeidis in soil. Trans. Br. Mycol. Soc., 87: 511-517.
- Attri, K., M. Sharma and S.K. Gupta, 2018. Influence of edaphic factors on Fusarium wilt of bell pepper. Int. J. Bioresour. Stress Manage., 9: 606-610.
- Alhussaen, K.M., 2012. Effect of soil acidity on diseases caused by Pythium ultimum and Fusarium oxysporum on tomato plants. J. Biol. Sci., 12: 416-420.
- Olutiola, P.O., 1978. Growth, sporulation and production of pectic and cellulolytic enzymes in Fusarium oxysporum. Trans. Br. Mycol. Soc., 70: 109-114.
- Aamir, M., S.P. Kashyap, V.K. Singh, M.K. Dubey, W.A. Ansari, R.S. Upadhyay and S. Singh, 2019. Trichoderma erinaceum bio-priming modulates the WRKYs defense programming in tomato against the Fusarium oxysporum f. sp. lycopersici (Fol) challenged condition. Front. Plant Sci., 10.
- Herath, H.H.M.A.U., R.L.C. Wijesundera, N.V. Chandrasekharan, W.S.S. Wijesundera and H.S. Kathriarachchi, 2015. Isolation and characterization of Trichoderma erinaceum for antagonistic activity against plant pathogenic fungi. Curr. Res. Environ. Appl. Mycol., 5: 120-127.
- Swain, H., T. Adak, A.K. Mukherjee, P.K. Mukherjee and P. Bhattacharyya et al., 2018. Novel Trichoderma strains isolated from tree barks as potential biocontrol agents and biofertilizers for direct seeded rice. Microbiol. Res., 214: 83-90.
- Rojo, F.G., M.M. Reynoso, M. Ferez, S.N. Chulze and A.M. Torres, 2007. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Protect., 26: 549-555.
- Lian, H., R. Li, G. Ma, Z. Zhao, T. Zhang and M. Li, 2023. The effect of Trichoderma harzianum agents on physiological-biochemical characteristics of cucumber and the control effect against Fusarium wilt. Sci. Rep., 13.
- Bokhari, N.A. and K. Perveen, 2012. Antagonistic action of Trichoderma harzianum and Trichoderma viride against Fusarium solani causing root rot of tomato. Afr. J. Microbiol. Res., 6: 7193-7197.
- Bhadra, M., A. Khair, M.A. Hossain and M.M. Sikder, 2015. Efficacy of Trichoderma spp. and fungicides against Lasiodiplodia theobromae. Bangladesh J. Sci. Ind. Res., 49: 125-130.
- Chen, S.C., J.J. Ren, H.J. Zhao, X.L. Wang and T.H. Wang et al., 2019. Trichoderma harzianum improves defense against Fusarium oxysporum by regulating ROS and RNS metabolism, redox balance, and energy flow in cucumber roots. Phytopathology, 109: 972-982.
- Zhang, Y., C. Tian, J. Xiao, L. Wei, Y. Tian and Z. Liang, 2020. Soil inoculation of Trichoderma asperellum M45a regulates rhizosphere microbes and triggers watermelon resistance to Fusarium wilt. AMB Express, 10.
- Al-Kahal, A.A., H.M. Mansoor and E.A. Ashmawy, 2009. Effect of copper oxychloride, elemental sulphur, and rhizobium inoculation on root rot disease, nodulation, and growth of faba bean plants. J. Soil Sci. Agric. Eng., 34: 5773-5783.
- Masoud, S.A., A.R. Emara and A.S. Mansy, 2022. Studying the efficiency of some nanoparticles on some plant pathogenic fungi and their effects on hyphal morphology. Iraqi J. Agric. Sci., 53: 1476-1485.
How to Cite this paper?
APA-7 Style
Sikder,
M.M., Akter,
P., Ahmmed,
M.S., Akter,
B., Alam,
N.B., Sajuti,
S., Alam,
M.N. (2024). Black Stem of Sunflower: An Emerging Fungal Disease Caused by Fusarium solani in Bangladesh. Asian Journal of Biological Sciences, 17(4), 709-719. https://doi.org/10.3923/ajbs.2024.709.719
ACS Style
Sikder,
M.M.; Akter,
P.; Ahmmed,
M.S.; Akter,
B.; Alam,
N.B.; Sajuti,
S.; Alam,
M.N. Black Stem of Sunflower: An Emerging Fungal Disease Caused by Fusarium solani in Bangladesh. Asian J. Biol. Sci 2024, 17, 709-719. https://doi.org/10.3923/ajbs.2024.709.719
AMA Style
Sikder
MM, Akter
P, Ahmmed
MS, Akter
B, Alam
NB, Sajuti
S, Alam
MN. Black Stem of Sunflower: An Emerging Fungal Disease Caused by Fusarium solani in Bangladesh. Asian Journal of Biological Sciences. 2024; 17(4): 709-719. https://doi.org/10.3923/ajbs.2024.709.719
Chicago/Turabian Style
Sikder, Md., Maniruzzaman, Popy Akter, Md. Sabbir Ahmmed, Beauty Akter, Nusrat Binte Alam, Sayma Sajuti, and Md. Nuhu Alam.
2024. "Black Stem of Sunflower: An Emerging Fungal Disease Caused by Fusarium solani in Bangladesh" Asian Journal of Biological Sciences 17, no. 4: 709-719. https://doi.org/10.3923/ajbs.2024.709.719

This work is licensed under a Creative Commons Attribution 4.0 International License.



