Effect of Probiotics-YUGE® on Survival and Growth of Heterobranchus bidorsalis Larvae Reared in Static-Renewal System
| Received 27 Dec, 2022 |
Accepted 28 Mar, 2023 |
Published 30 Jun, 2023 |
Background and Objective: The poor survival and growth of Heterobranchus bidorsalis larvae recorded during rearing from post-yolk absorption to fingerlings in the hatchery have been a major problem brothering farmers in Northern Nigeria. This study was conducted to evaluate the efficacy of a commercial diet supplemented with commercial probiotics YUGE® in the rearing of Heterobranchus bidorsalis larvae to test if it will improve survival and growth in a static renewal system. Materials and Methods: Two weeks old larvae weaned on decapsulated artemia were reared on 0.0, 1.0, 2.0 and 3.0 g kg‾1 levels of probiotics YUGE® supplemented commercial diet (Aqualis® fry powder) making up four dietary treatments allocated in triplicate in a completely randomized design. Results: Survival, growth and microbial constituent of larvae subjected to each treatment were monitored during four weeks of rearing. The result of the study showed that there was no significant difference (p>0.05) in the percent survival rate among the treatments. Larvae-fed probiotics YUGE® supplemented at 1.0 to 2.0 g kg‾1 revealed higher growth and the highest Bacillus spp., recorded in gut microflora. Conclusion: It was concluded that the supplementation of the probiotics YUGE® in the diets of the larvae enhanced the percent occurrence of gut-beneficial Bacillus spp., at 1 to 2 g kg‾1 of each which could have influenced the larvae’s better performance in terms of survival and growth.
| Copyright © 2023 Abubakar and Magawata. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The sustainability of any aquaculture venture depends on several factors that include the availability of good quality seed and a foot greatly influenced by the availability of the right and less costly feed to attain the survival of larvae up to fingerlings. Starter feeds are increasing in cost by the day and continued trials of available supplements to ease feed utilization and enhance good feed conversion and survival become necessary. In Nigeria, catfish of the family Clariidae are widely distributed and the most cultured. However, the survival, growth and development during the nursery phase of larvae to juveniles are faced with a lot of mortality and poor growth. According to the research1-3, before the larval period of development, larvae nutrition has been provided by the yolk sac and oil globule and after this, the organs are developed and function much as those in an adult fish. The transition from an endogenous to an exogenous food supply which marks the onset of the larval stage is one of the most critical phases of the life cycle and is the period when much of the mortality of hatchery-reared stock occurs. The requirement for a particular nutrient can be defined from a physiological point of view as the nutrient intake needed to fulfill a physiological role4. Fish require diets that are balanced in energy, protein, minerals and vitamins at all stages of development. The survival and growth rate of larvae during rearing is mostly influenced by the quality of feed and its acceptability, especially during intensive rearing5, feeding constitutes a major factor since the fish obtain a good percentage of their nutritional requirements through the food they consume. The acceptability of feed depends on the feed type and particle size. Hence, an improvement in the artificial feed provided to ensure fry survival becomes very important. Nowadays, probiotics YUGE® have been widely used in aquaculture for the control of disease and also to increase feed efficiency and husbandry parameters6. The beneficial effects of these probiotics include higher growth and better feed efficiency, prevention of intestinal disorders and pre-digestion of anti-nutritional factors present in the ingredients. This study, therefore, tried to establish the influence of commercial probiotics YUGE® on the growth performance and intestinal microbial flora of H. bidorsalis larvae and which level of application in feed best enhances survival and growth performance in the hatchery.
MATERIALS AND METHODS
Study area: The experiment was carried out from June to July in the year 2017. This study was carried out at the Teaching and Research Fish Farm, Department of Fisheries and Aquaculture, Usmanu Danfodiyo University Sokoto. The site is on Latitude 13°07'78"N and Longitude of 05°12'25"E at 275 m above sea level. The site is located in the Sudan Savanah of Nigeria, with an agro-climate characterized by seven long dry months, occurring from October to April of every year, mean monthly maximum temperature of 31-40°C and mean monthly minimum temperature of 12-24°C and evapotranspiration of the order 1670 mm. The area is characterized by cool dry air during the harmattan from November to February and the hot season from March to May. Annual rainfall in the area ranged from 508 to 1016 mm/year7. The mean relative humidity is 14.9 and 40% in March and June, respectively.
Probiotics: The probiotic’s composition and the manufacturer’s detail were presented in Table 1.
Fish larvae: Larvae of the fish weaned from decapsulated artemia as starter feeds for two weeks in the teaching and research hatchery of the Department of Fisheries and Aquaculture, Usmanu Danfodiyo University Sokoto, were sampled and subjected to a feeding trial.
Experimental design, data collection and analysis: The experiment was carried out in 12 experimental units (70-liter capacity plastic bowls filled with clean water and fitted with a mechanical aerator) in a static renewal system (water exchange every 24 hrs). One hundred and fifty larvae (fry) were randomly sampled and stocked in each experimental unit. Diets (Aqualis® fry powder) supplemented with varying levels of YUGE®(0.0, 1.0, 2.0 and 3.0 g kg–1) make up the dietary treatments (the manufacturer’s recommended level of YUGE® for freshwater fish is 1-2 g kg–1 of feed) and allocated in triplicate making up to 4 experimental treatments arranged in a completely randomized design. The procedures applied in the data collection, data analysis and statistical analysis were carried out following the procedure by Abubakar and Ipinjolu8
| Table 1: | Name and manufacturer of probiotics used in this study | |||
| Probiotics | Composition | Manufacturer |
| YUGE® | Bacillus subtilis: 200 million CFU g–1 | Shandong Baolai-Leelai Bio-Tech., Co., Ltd., China |
| Pediococcus acidilactici: 100 million CFU g–1 | ||
| Maifanite: 40% | ||
| Starch: 35% | ||
| Fermented Aspergillus oryzae: 20% | ||
| Vitamin C: 5% | ||
| Total viable bacteria: >300 million CFU g–1 |
Statistical analysis: Data collected on growth, survival, feed utilization and intestinal microflora were subjected to Analysis of Variance (ANOVA) and means were separated using New Duncan’s Multiple Range Test (DMRT)9. Computer analysis was carried out using the SPSS V: 20.0 package for Windows.
RESULTS
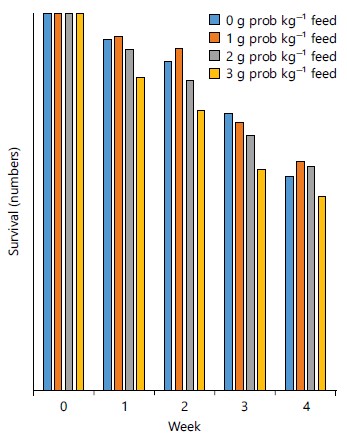
The percent survival rate was not significantly (p>0.05) different among the four dietary treatments where those fed Treatment II had 60% and the least 51% was in Treatment IV, as presented in Table 2. The trend in the survival of the larvae in (Fig. 1) showed that larvae fed dietary treatment II (1 g kg–1), maintained the highest mean survival (141, 136 and 91) throughout the experimental period (weeks 1 to 4) respectively, followed by those fed dietary treatment I (0 g kg–1) except in the third week where that fed dietary treatment I, had the highest survival average of (110). The groups placed on dietary treatment III (2 g kg–1 feed) had low mortality with a mean survival rate of (89) in the 4th week (Fig. 1) of the experiment and had better survival than those on the treatment I and IV
| Table 2: | Survival and growth indices of Heterobranchus bidorsalis larvae fed varying levels of probiotics YUGE® for 28 days | |||
Treatment/(g) probiotics/(kg) feed |
||||
| Parameter | TRT I (0 g kg–1) |
TRT II (1 g kg–1) |
TRT III (2 g kg–1) |
TRT IV (3 g kg–1) |
| Initial fish number | 450 |
450 |
450 |
450 |
| Final fish number | 256 |
274 |
267 |
232 |
| Survival rate (%) | 56.89±10.11 |
60.89±9.56 |
59.33±3.01 |
51.56±2.22 |
| Initial body weight (mg) | 67.78±0.44 |
67.56±0.97 |
68.22±0.22 |
67.77±0.44 |
| Final body weight (mg) | 423.49±40.87b |
526.99±33.65ab |
626.67±20.28a |
487.21±47.51b |
| Weight gain (mg) | 355.71±40.43b |
459.44±34.62ab |
558.44±20.26a |
419.43±47.18b |
| Weight gain (%) | 524.09±55.87b |
681.83±60.72ab |
818..57±29.72a |
618.22±66.70b |
| Specific growth rate (%/day) | 6.51±0.31b |
7.32±0.49ab |
7.92±0.20a |
7.01±0.12ab |
| Initial body length (mm) | 19.33±0.88 |
19.00±0.58 |
18.67±0.33 |
19.67±0.33 |
| Final body length (mm) | 44.00±2.52 |
47.33±2.96 |
48.00±0.58 |
47.00±0.99 |
| Length increase (mm) | 24.67±3.18 |
28.33±3.53 |
29.33±0.33 |
27.33±1.45 |
| Length increase (%) | 129.34±21.92 |
150.52±23.32 |
157.21±2.47 |
139.30±9.74 |
| Condition factor (k) | 0.52±0.11 |
0.51±0.08 |
0.57±0.04 |
0.48±0.08 |
| Mean values in row with same letter are not significantly different (p>0.05), TRT: Treatment | ||||
|
|
The growth responses of the larvae are presented in Table 2. Larvae fed diet III (2 g kg–1) had the highest weight gain (558.44±20.26) with no significant difference (p>0.05) from those fed diet II (1 g kg–1) (459.44±34.62), but significantly (p<0.05) higher than the mean weight gains of 355.71±40.43 and 419.43±47.18 obtained for larvae fed diets I (control) and IV (3 g kg–1), respectively. However, there was no significant (p>0.05) difference in the weight gained among larvae fed diets I, II and IV. The percent weight gained also followed the same trend. The specific growth rate (SGR (%)/day) indicates that larvae fed diet III (2 g kg–1) had 7.92±0.20 which was significantly not different from those fed diets II (1 g kg–1) and IV (3 g kg–1). The control treatment recorded the least SGR but was not significantly different (p>0.05) from those fed diets II and IV. Results of the length increase and condition factor recorded were non-significantly (p>0.05) differences among the larvae subjected to the four dietary treatments.
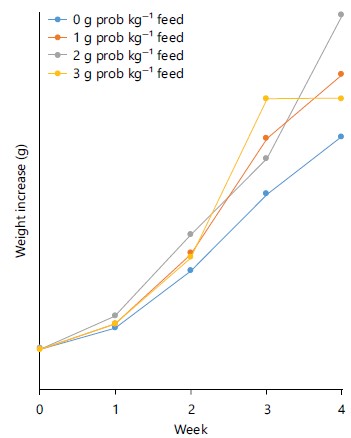
The trend in the growth of the larvae on the different YUGE®-supplemented dietary treatments is as presented in Fig. 2. The graph showed that larvae fed dietary treatment IV (3 g kg–1), maintained a competitive growth rate with those fed dietary treatment III and achieved the highest growth rate (42,426 mg) during the third week and declined in the fourth week of the experiment to (37,911 mg) when compared to other dietary treatments (II and III) that recorded higher weight increase 47,174 and 55,943 mg, respectively. However, larvae fed dietary treatments II and III maintained a competitive growth performance through the period of the trial but the latter showed better performance with increased weight gain than those on diet II (1 g kg–1). Those fed the control dietary treatment I (0 g kg–1) maintained the least weight gain throughout the testing period.
Results of the water quality parameters (temperatures, pH, dissolved oxygen and ammonia) during the period of the probiotic YUGE® experiment were presented in Table 3. The overall mean of the water temperature during the experimental period was 30.19±0.34°C with minimum and maximum values of 28.4 and 32°C respectively. The temperature varied from 27.3 to 30.9, with a mean of 29.07±0.33 in the morning, 29.5 to 32.1 with a mean of 31.06±0.25 in the afternoon and 28.4 to 33 with a mean of 30.44±0.45 in the evening. The mean pH during the experimental period was 7.75±0.09 and the value ranged from 7.35 to 8.23. The mean and the range of dissolved oxygen and ammonia concentrations were 4.67±0.20 (4.03-4.18) and 0.093±0.04 (0.088-0.096), respectively. There were no significant differences among the means of the water quality parameters among the four treatments.
| Table 3: | Mean water quality parameters measured during probiotics YUGE® experimental period | |||
| Parameter | Mean |
Minimum |
Maximum |
| Temperature (°C) | |||
| Morning | 29.07±0.33 |
27.3 |
30.9 |
| Afternoon | 31.06±0.25 |
29.5 |
32.1 |
| Evening | 30.44±0.45 |
28.4 |
33 |
| Overall mean | 30.19±0.34 |
28.4 |
32 |
| PH | 7.75±0.09 |
7.35 |
8.23 |
| Dissolved oxygen (mg L–1) | |||
| TRT I | 4.62±0.32 |
4.62 |
5.39 |
| TRT II | 4.75±0.21 |
4.02 |
5.33 |
| TRT III | 4.61±0.15 |
4.22 |
5 |
| TRT IV | 4.71±0.13 |
4.25 |
5 |
| Overall mean | 4.67±0.20 |
4.03 |
4.18 |
| Ammonia (mg L–1) | |||
| TRT I | 0.93±0.01 |
0.89 |
0.96 |
| TRT II | 0.94±0.02 |
0.87 |
0.98 |
| TRT III | 0.90±0.12 |
0.86 |
0.95 |
| TRT IV | 0.94±0.01 |
0.9 |
0.96 |
| Overall mean | 0.93±0.04 |
0.88 |
0.96 |
| Mean values in row with same letter are not significantly different (p>0.05) and TRT: Treatment | |||
| Table 4: | Mean number of cells (g–1 CFU) in the gut of Heterobranchus bidorsalis 28 days after feeding with | |||
Bacterial count (CFU g–1) |
|||
| Treatment | Mean count |
Minimum |
Maximum |
| Initial | 4.3±0.6×105 |
3.0×105 |
5.0×105 |
| TRT I (Control) | 7.3±2.4×105 |
4.0×105 |
12.0×105 |
| TRT II (1 g YUGE® kg–1 of feed) | 5.3±1.7×105 |
2.0×105 |
8.0×105 |
| TRT III (2 g YUGE® kg–1 of feed) | 22.3±1.7×105 |
5.0×105 |
57.0×105 |
| TRT IV (3 g YUGE® kg–1 of feed) | 7.3±2.0×105 |
4.0×105 |
11.0×105 |
| Mean values in row with same letter are not significantly different (p>0.05) and TRT: Treatment | |||
| Table 5: | Frequency of occurrence of bacterial isolates | |||
| Bacterial isolate | Frequency of occurrence |
Occurrence (%) |
Bacterial type (%) |
|
| Proteus vulgaris | 1 |
6 |
||
| Salmonella typhi | 1 |
6 |
Bacillus spp. |
42 |
| Citrobacter fruendii | 1 |
6 |
||
| Bacillus firmus | 2 |
12 |
||
| Bacillus subtilis | 3 |
18 |
||
| Salmonella paratyphia | 1 |
6 |
Other bacterial |
58 |
| Listeria monocytogenes | 3 |
18 |
||
| Micrococcus kristinae | 1 |
6 |
||
| Kurthia spp. | 1 |
6 |
||
| Bacillus lentus | 2 |
12 |
||
| Morganella morganii | 2 |
12 |
||
| Total | 18 |
100 |
100 |
The mean bacterial counts and frequency of occurrence of bacterial species isolated from the larvae gut fed different dietary treatments containing probiotics YUGE® are presented in Table 4 and 5. The initial bacterial counts from the larvae before subjecting them to probiotics YUGE® was observed to be lower than 4.3±0.6×105 and this was lower than the load counted after feeding them probiotics YUGE®. The highest mean count obtained after the trial was in treatment II (1 g YUGE® kg–1 of feed) (22.3±1.7×105) followed by those in treatments I (Control) and IV (3 g YUGE® kg–1 of feed) with 7.3±2.4×105 and 7.3.0±2.0×105, respectively, while the least was in treatment II (1 g YUGE® kg–1 of feed) 5.3±1.7×105.
The total number of bacteria counts in larvae subjected to probiotic YUGE® supplemented diets was eighteen. Bacillus subtilis and Listeria monocytogenes accounted for the highest frequency of 18% followed by the occurrence of Bacillus firmus, Bacillus lentus and Morganella morganii with 12% each. The least occurring were Proteus vulgaris, Salmonella typhi, Citrobacter fruendii, Salmonella paratyphi A, Micrococcus kristinae and Kurthia spp., each with 6% occurrence. The Bacillus spp., accounted for 42% while other bacterial groups scored 58% of the total occurrence.
DISCUSSION
The growth and survival of H. bidorsalis larvae on probiotics YUGE®, showed that there was no significant difference (p>0.05) in percent survival rate among the treatments. The survival trend observed in this study was observed to be similar to the trend observed by Pooramini et al.10. The best weight-gain obtained in TRT II in this study could be influenced by the microbial floral change, where bacillus species were recorded to be highest in the gut of larvae fed 2 g kg–1 YUGE® and evidence of dominant colonization. This was similar to the findings of researchers11-13 where administration of probiotics significantly changed the proportion of Bacillus bacteria in the gut flora of freshwater fish. Bairagi et al.14, showed that two specific strains of fish intestinal bacteria, Bacillus subtilis and B. circulans, having extracellular cellulolytic and amylolytic activities, were used to inoculate duckweed (Lemna polyrhiza) leaf meal and had the best effects on the growth and feeding efficiency of rohu fingerlings. The early intestinal colonization of the gut of a fish by the probiotic may have some effect on development, by accelerating the maturation of the digestive system and stimulating metabolism and growth12,15. The presence of more Bacillus spp., which is known to produce digestive proteases and other enzymes that enable it to contribute to the natural digestion activity of the cultured species and it can be a source of micro and macro-elements as feed16-18 up to 42% (Table 5), could have enhanced better growth recorded in the larvae fed probiotics YUGE® supplemented diet. The sudden drop in the growth (Fig. 2) of larvae subjected to dietary treatment IV (3 g kg–1) in the fourth week could be a sign of a limit in the level of exposure of the larvae to the high level of probiotics YUGE® supplement. This buttresses the finding of Bagheri et al.19, who observed that the presence of the largest probiotics YUGE® cells in diets and host intestine necessarily does not result in the ppdimproved growth and survival of Onchorrhynchus mykiss fry. This further confirms the necessity for proper supplementation of the correct probiotics YUGE® quantity to function effectively in the diets of fish larvae.
Implications: To ensure effective supplementation of basal diet with probiotics YUGE®, it is recommended that H. bidosalis fry be fed at 1 to 2 g kg–1, for better utilization of basal feed and stimulation of productive performance. The results of this investigation will be helpful in formulation of practical diets for culture of fry.
CONCLUSION
The findings from the study showed that different levels of probiotics YUGE® could cause different effects on growth parameters in Heterobranchus bidorsalis larvae. Better growth was obtained in larvae fed at 1 and 2 g kg–1 of basal feed. It can be concluded that the supplementation of the probiotics YUGE® in the diets of the larvae enhanced the percent occurrence of gut beneficial Bacillus spp., at 2 g kg–1 which could have influenced the larvae performance in terms of survival and growth when reared in a static renewal water exchange system.
SIGNIFICANCE STATEMENT
This study discovered that probiotics YUGE® can be beneficial for enhancing the survival and growth of Heterobranchus bidorsalis post yolk absorption larvae in a static renewal water exchange system. This study will help the researchers to uncover the critical areas of probiotics YUGE® that many researchers were yet able to explore on its efficacy on Heterobranchus bidorsalis.
REFERENCES
- Olaniyi, W.A. and O.G. Omitogun, 2014. Embryonic and larval developmental stages of African giant catfish Heterobranchus bidorsalis (Geoffroy Saint Hilaire, 1809) (Teleostei, Clariidae). SpringerPlus, 3: 677.
- Abubakar, M.Y. and J.K. Ipinjolu, 2018. Performance of Heterobranchus bidorsalis larvae fed artificial starter diets. Afr. J. Resour. Manage. Fish. Aquat., 3.
- Adewolu, M.A., S.L. Akintola and O.O. Akinwunmi, 2009. Growth performance and survival of hybrid African catfish larvae (Clarias gariepinus X Heterobranchus bidorsalis) fed different diets. Zoologists, 7: 45-51.
- Holt, G.J., 2011. Larval Fish Nutrition. John Wiley and Sons, Inc., Hoboken, New Jersey, ISBN: 9780470959862, Pages: 436.
- Hamre, K., M. Yúfera, I. Rønnestad, C. Boglione, L.E.C. Conceição and M. Izquierdo, 2013. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquacult., 5: S26-S58.
- Suzer, C., D. Çoban, H.O. Kamaci, S. Saka, K. Firat, O. Otgucuoğlu and H. Küçüksari, 2008. Lactobacillus sp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: Effects on growth performance and digestive enzyme activities. Aquaculture, 280: 140-145.
- Umar, A.T. and M.M. Bako, 2019. Recent rainfall trends and variability in Sudano-Sahelian Region of Nigeria (1986- 2015). Ghana J. Geogr., 11: 33-57.
- Abubakar, M.Y. and J.K. Ipinjolu, 2020. Survival and growth performance of Heterobranchus bidorsalis larvae fed diet supplemented with e-probiotic 111®. J. Aquacult. Mar. Biol., 9: 71-78.
- Gomez, K.A. and A.A. Gomez, 1984. Statistical Procedure for Agricultural Research. 2nd Edn., John Wiley and Sons, Hoboken, New Jersey, ISBN: 9780471870920, Pages: 704.
- Pooramini, M., A. Kamali, A. Hajimoradloo, A. Alizadeh and R. Ghorbani, 2009. Effect of using yeast (Saccharomyces cerevisiae) as probiotic on growth parameters, survival and carcass quality in rainbow trout Oncorhynchus mykiss fry. Int. Aquat. Res., 1: 39-44.
- Salinas, I., A. Cuesta, M.A. Esteban and J. Meseguer, 2005. Dietary administration of Lactobacillus delbrueckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol., 19: 67-77.
- Avella, M.A., G. Gioacchini, O. Decamp, P. Makridis, C. Bracciatelli and O. Carnevali, 2010. Application of multi-species of Bacillus in sea bream larviculture. Aquaculture, 305: 12-19.
- Yanbo, W. and X. Zirong, 2006. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim. Feed Sci. Technol., 127: 283-292.
- Bairagi, A., K.S. Ghosh, S.K. Sen and A.K. Ray, 2002. Duckweed (Lemna polyrhiza) leaf meal as a source of feedstuff in formulated diets for rohu (Labeo rohita Ham.) fingerlings after fermentation with a fish intestinal bacterium. Bioresour. Technol., 85: 17-24.
- Ringø, E., R. Harikrishnan, M. Soltani and K. Ghosh, 2022. The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals, 12: 3016.
- Verschuere, L., G. Rombaut, P. Sorgeloos and W. Verstraete, 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev., 64: 655-671.
- Ziaei-Nejad, S., M.H. Rezaei, G.A. Takami, D.L. Lovett, A.R. Mirvaghefi and M. Shakouri, 2006. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture, 252: 516-524.
- Jafaryan, H., M.M. Taati and M. Jafarzadeh, 2011. The enhancement of growth parameters in common carp (Cyprinus carpio) larvae using probiotic in rearing tanks and feeding by various Artemia nauplii. Aquacult. Aquarium Conserv. Legislation Int. J. Bioflux Soc., 4: 511-518.
- Bagheri, T., S.A. Hedayati, V. Yavari, M. Alizade and A. Farzanfar, 2008. Growth, survival and gut microbial load of rainbow trout (Onchorhynchus mykiss) fry given diet supplemented with probiotic during the two months of first feeding. Turk. J. Fish. Aquat. Sci., 8: 43-48.
How to Cite this paper?
APA-7 Style
Abubakar,
M.Y., Magawata,
I. (2023). Effect of Probiotics-YUGE® on Survival and Growth of Heterobranchus bidorsalis Larvae Reared in Static-Renewal System
. Asian Journal of Biological Sciences, 16(2), 187-194. https://doi.org/10.3923/ajbs.2023.187.194
ACS Style
Abubakar,
M.Y.; Magawata,
I. Effect of Probiotics-YUGE® on Survival and Growth of Heterobranchus bidorsalis Larvae Reared in Static-Renewal System
. Asian J. Biol. Sci 2023, 16, 187-194. https://doi.org/10.3923/ajbs.2023.187.194
AMA Style
Abubakar
MY, Magawata
I. Effect of Probiotics-YUGE® on Survival and Growth of Heterobranchus bidorsalis Larvae Reared in Static-Renewal System
. Asian Journal of Biological Sciences. 2023; 16(2): 187-194. https://doi.org/10.3923/ajbs.2023.187.194
Chicago/Turabian Style
Abubakar, Mohammad, Yahaya, and Ibrahim Magawata.
2023. "Effect of Probiotics-YUGE® on Survival and Growth of Heterobranchus bidorsalis Larvae Reared in Static-Renewal System
" Asian Journal of Biological Sciences 16, no. 2: 187-194. https://doi.org/10.3923/ajbs.2023.187.194

This work is licensed under a Creative Commons Attribution 4.0 International License.



