Chemical and Pharmacological Activities of Rape Seed Mustard Brassica nigra (Black Mustard): A Magic Herb
| Received 15 Jul, 2024 |
Accepted 23 Oct, 2024 |
Published 31 Mar, 2025 |
Brassica nigra (Black mustard) is a much-branched annual herb belonging to the family Brassicaceae. This plant is native to Mediterranean Region and various other countries like India and Europe. Brassica nigra possesses many pharmacological effects. It is full of minerals and vitamins, including A, K and C, as well as other components found in mustard greens. A good source of iron, calcium, magnesium and other minerals are mustard seeds. Because they have an almost 1:1 ratio of omega 3 to 6 fatty acids, they are also a good source of omega-6 fatty acids. ManyBrassica nigra extracts and their isolated seed components have been shown to exhibit a variety of pharmacological characteristics, such as antioxidant, anti-inflammatory, anticancer, antibacterial and neuroprotective effects. Leaf and dried seed extracts from Brassica nigra exhibit a wide range of pesticidal properties, such as insecticidal, nematicidal and molluscicidal effects. Consumption of Brassica nigraextracts by humans and animals has the advantage to put off in vivo oxidative damage linked with diseases and illnesses. Thus, the present review paper provides a detailed number of facts reported by scientists about the nutritional value and pharmacological activities associated with the Brassica nigra.
| Copyright © 2025 Singh and Singh. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
According to Sanskrit records from approximately 3000 BC, mustard is one of the earliest spices known to exist1. It was also one of the first crops to be domesticated. Three varieties of mustard seeds are commonly utilized as condiments: Brassica nigra (black or dark brown mustard), brown or oriental mustard (Brassica juncea) and pale yellow or white mustard (Sinapis alba syn. Brassica hirta Moench or Brassica alba). According to Farrell2, Sinapis alba is currently grown extensively across Australia, China, Chile, Denmark, Italy, Japan, the UK, Netherlands, North Africa, Canada and the USA. Brassica juncea (L.) Czern and Coss Brown mustard was originally introduced from China into Northern India from where it has extended to Afghanistan Via Punjab3.
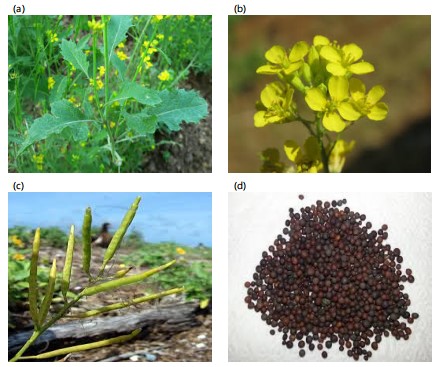
Brassica nigra (Linn.) Koch’s syn. true mustard is made from black mustard seeds. It is likely native to the Southern Mediterranean region. In addition to its significance as a crop plant, Brassica nigra has aided in the evolution of other species within the Brassica genus. Black mustard or Brassica nigra, seeds are authentic mustard. Local names for the plant include rai (Hindi), senafich (Amharic), zwartemosterd (Dutch) and mostazanegra (Spanish). It is extensively grown all over the world as the main source of edible oil and medication4-7. It belongs to the genus Brassica and has distinct genetic ancestor genes8. In addition to being a valuable crop, it helped other species in the Brassica genus evolve. It is believed that Brassica nigra originated mostly in the Mediterranean region of Central and Southern Europe. It is grown as a cool season garden crop on a small scale in Punjab, Delhi, Utter Pradesh, Madhya Pradesh and Western and Southern India mainly for its seeds which are consumed as a condiment. Black mustard is an annual herbaceous plant growing to between 0.6 and 1.2 m. Leaves are dark green, petiolate, alternate and hairy (Fig. 1a). Large leaves are rough, irregularly sinuate-dentate and pinnate with terminal lobe large and small lower lobes arranged at the lower side of the plant (Fig.1b). Smooth and moderately lobed leaves are arranged at the upper side of the plant. The tiny, brilliant yellow, hermaphrodite flowers have four petals in a cruciform shape, tetradynamus stamens, a bicarpellate pistil and are pollinated by flies and bees. The fruit has a smooth texture, siliqua, quadrangular shape and green colour (Fig. 1c). The appearance of dry mustard seeds is different from that of other varieties; they are tiny, reddish-brown to black (Fig. 1d). Little seeds are 2 mm in size, slightly more oblong than spherical, vary in colour and have a white pellicle covering them. They also smell far stronger than white seeds.
|
In the United States of America or Europe black mustard is not a common crop because of problems in harvesting. It grows in acidic, basic (alkaline) and neutral soil and also grows in very acidic soil. Brassica nigra seeds are used as spice condiments. Seed contains a significant amount of fatty oil, which is used as cooking oil and also used to treat respiratory infections9. This plant has important medical uses in the treatment of many diseases, including joint pain, liver diseases, rheumatism, as well as the spleen, oral as well as tumours in addition to being a laxative10. It is mainly grown for oil extraction, food, green fodder and animal cake production11. It has been reported as a potential Phyto extractant and metal accumulator and it tolerates the presence of heavy metal concentrations in the soil12,13.
|
| Table 1: | Chemical composition of Brassica nigra (Per 1 cup [approx. 56 g] chopped mustard greens, raw) | |||
| Nutrients profile | Nutrients | Calories |
| Macronutrient profile: (Per 1 cup [approx. 56 g] | 1.6 g protein | 1.6 g |
| chopped mustard greens, raw) | 2.6 g carbohydrate | 2.6 g |
| 0.2 g fat | 0.2 g | |
| Secondary metabolites: (Per 1 cup [approx. 56 g] | Vitamin K | 144.2 mcg (180.3% DV) |
| chopped mustard greens, raw) | Vitamin C | 39.2 mg (65.3% DV) |
| Vitamin A | 1,693 IU (33.9% DV) | |
| Manganese | 0.3 mg (15% DV) | |
| Dietary fiber | 1.8 g (7.2% DV) | |
| Calcium | 64 mg (6.4% DV) | |
| Potassium | 215 mg (6.1% DV) | |
| Vitamin E | 1.13 mg (5.6% DV) | |
| Iron | 0.92 mg (5.1% DV) |
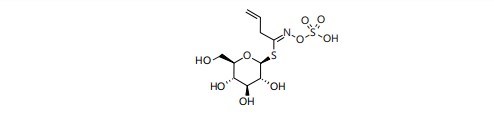
Chemical constituents: Sinapis alba (yellow mustard) contains the glucosinolase sinalbin but Brown mustard (Brassica juncea) and Black mustard (Brassica nigra) containcontain glucosinolate, sinigrin (potassium myronate) and the enzyme myrosin (myrosinase) are the most significant components. Other components include fixed oils (25-37%), which are primarily made up of glycerides of erucic, eicosenoic, arachidic, nonadecanoic, behenic, oleic and palmitic acids14. A thioglycoside like compound of ally isothiocyanate with glucose (allylglucosinolate) found in black mustard contains about 1% sinigrin (Fig. 2)15. An amino acid-derived side chain, a sulfonated oxime group and a thioglucose group Glucosinolates (GSLS) are plant hydrophilic secondary metabolites, commonly found in the order Brassicales16. In different quantities and types, these compounds are produced in many plant tissues and organs.
The Allyl isothiocyanate, a pungent and volatile compound, is liberated by the action of the enzyme myrosinase. Besides allyl isothiocynate, Crotonyl isocyanate (2-butenylisothiocyanate), another related compound is found in Romanian Brown Mustard. Analysis of sample of seed gave the following values: Moisture, 8.5; protein, 20; fat, 39.7; fibre, 1.8; other carbohydrate, 23.8; minerals, 4.2; calcium 490; phosphorus, 700; iron, 17.9; thiamine, 0.65; riboflavin, 0.26 and niacin, 4.0 mg/100 g and carotene 152/100 g (Table 1)4. The seed also contains choline (211 mg/100 g) and ascorbigen17. The presence of tertiary and quaternary alkaloids has been reported in the aqueous extract of the seed and roots. Hungarian and Chilean seeds contain 0.6 and 1.260% volatile oil, respectively. The seed also contains lecithin (0.63-1.04%). Like the seeds, mustard seeds contain also a significant amount of fatty oil (30%), which is used extensively for cooking in India; furthermore, traces of free isothiocyanates may be found in mustard oil. alkaloids, saponins and tannins are present only in B. nigra and B. juncea seeds, besides glycerides of linoleic and linolenic acid, mustard oil contains glycerides of erucic acid, which is considered harmful to human health18-20.
Pharmacological effects: The majority of population in Asia and Africa depends primarily on traditional medicine that mainly involves the use of plant extracts21. More than 700 are noted for their uses as medicinal herbs in total 2600 plant species22. Different plant products and medicinal herbs were used in treating a broad spectrum of infections and other diseases in folk medicine23-26. Parts of B. nigra have been used in therapeutic preparations for centuries in different parts of world. Seed of Brassica nigra is often used in herbal medicine, especially as a rubefacient poultice27. Mustard seed is ground and made into a paste then applied to the skin and acts as a laxative. Seeds are used in the treatment of in duration of the spleen and liver. The seed is eaten as a tonic28-30. Reducing congestion in internal organs in the treatment of rheumatism31. Mustard relieves congestion by drawing the blood to the surface as in head afflictions, neuralgia and spasms and possesses cognitive enhancing, antimigraine and anti-Parkinson’s properties. The seed stimulates the digestive secretions. Mustard was used for treating alopecia and epilepsy, appetizer, digestive, diuretic, emetic and tonic32.
Cytotoxicity: Brassica nigra extracts showed cytotoxic activity against five cancer cell lines, namely Hela (cervical carcinoma cells), HCT (colon carcinoma cells), HEPG2 (human cell line of a well-differentiated hepatocellular carcinoma isolated from a liver) MCF-7 (breast carcinoma cells) and Hep2 (Human epidermoid larynx carcinoma cells) using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)33. Cytotoxicity was determined by nonradioactive, colorimetric assay using the 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method.
Anticancer properties: In brassicales glucosinolates are amino acids derived secondary metabolites known to possess anti-cancer properties. In the area of health benefits, Glucoraphanin is the most widely investigated glucosinolate, healing properties of sulphoraphane, in cervical, breast, stomach, prostrate and colon cancer are well established. A diet rich in sulphoraphane can fight against Helicobacter pylori causing stomach ulcers. Sulphoraphane can also protect against rhinitis, arthritis, asthma, cystic fibrosis, aging and other lung disorders. Hence regular consumption of mustard products is highly recommended34-40. It is well recognized that Brassica spp. have the ability to prevent cancer and treat a variety of cancer forms, including breast, colon, ovarian, lung and bladder cancer41-44. Brassica nigra extracts in ethanolic, hexane and ethyl acetate have been shown to have antiproliferative properties against human cells from the hepatic (HepG2), cervical (HeLa), colorectal (HCT) and breast cancer (MCF-7) tissues45. Additionally, it demonstrated strong growth inhibitory effects on HeLa, HepG2, HCT, HEp2 and MCF-7 tumor cells46 including indole-3-carbinol, which prevents cancer cells from growing47-49. Its antiproliferative activity in liver tissue suggests that it could be useful in reducing the incidence of liver cancer50.
Anti-inflammatory effect: It is reported the analgesic activity of aqueous and ethanolic extracts of Brassica nigra. Acetic acid causes pain by liberating endogenous substances such as serotonin, histamine, prostaglandins (PGs), bradykinins and substance P from the nerve endings51. The suppression of inflammatory mediator release may account for the analgesic action of B. nigra extract. In comparison to the group treated with aspirin (100 mg/kg p.o.), the ethanolic extracts of B. nigra seeds demonstrated a better reduction of paw edema during the first and second hours. Its ethanolic and aqueous extracts have strong anti-inflammatory and analgesic properties51. The ethanolic extracts of Brassica nigra exhibited anti-inflammatory efficacy similar to that of phenylbutazone. Even while the extracts significantly reduced paw edema, their percentage of inhibition (17.90%) was still significantly lower than that of the usual medication, phenylbutazone, which reduces paw edema by 39.38%52. It is evident that the methanol extract of B. nigra leaves exhibits protective effect against d-GalN-induced hepatic and renal injury. Biochemical observations were supported by histological examinations of the liver and kidney. Based on the antioxidant and anti-inflammatory effects of extract from B. nigra leaves it may be suggested as a remedy in treatment of hepatic and renal injury53.
Anti-microbial activity: Brassica nigra seed was shown to be effective in reducing the fungus Aspergillus fumigatus a number of viable planktonic and sessile cells of a Pseudomonas spp. and a mixed sulphate-reducing bacteria (SRB) culture, all isolated from contaminated diesel oil. Only 1 hr treatment with 0.25% mustard seeds on the sessile state SRB were the most resistant, their number being reduced significantly54,55. It was tested for pathogen suppression and release of isothiocyanate and fungitoxic volatile produce in mustard tissue. In bioassays, 28 genotypes of B. nigra were screened for inhibition of the potato pathogens Verticillium dahliae and Helminthosporium solani by volatiles released from leaf tissue. Mustard produces compounds that suppress the radial growth of both fungi. Mustard treatment releasing >1.2 mg AITC/g plant tissue was fungicidal to both pathogens56,57.
| Table 2: | Result of antibacterial activity of mustard seed | |||
| Bacterium strains | Inhibition zone (mm)(30-g/disk) |
| Klebsiella pneumoniae FML 5 | 11 |
| Enterococcus faecalis ATCC 9345 | 15 |
| Micrococcus luteus ATCC 9345 | 19 |
| Staphylococcus aureus ATCC25923 | 15 |
| Bacillus megaterium DSM 32 | 8 |
| Pseudomonas aeruginosa ATCC25853 | 10 |
| Escherichia coli ATCC 11239 | 10 |
The antimicrobial activity of mustard seeds was shown in (Table 2). At the end of the antimicrobial activity tests, it is determined that mustard seeds have inhibitory activity in all test strains58.
If administered in low quantities, the use of Brassicaceae in the diet of bees has an intriguing effect on the suppression of Nosema ceranae and may have nutraceutical effects related to the bee’s lifetime59. According to Bhatia and Sharma60, B. nigra showed growth inhibitory effects against three bacterial strains, Pseudomonas aeruginosa, Shigella sonnei and Bacillus cereus, at various dose levels. Additionally, Senanayake et al.61 reported the activity against Rhizopus sp. and Aspergillus niger. On the other hand, Jasim62 showed how Brassica nigra seed oil extracts could combat harmful oral microflora. Strong antibacterial properties of Brassica nigra have been demonstrated by ethanolic extract. It was discovered that the extract exhibited activity against Streptococcus pyogenes, the causative agent of numerous significant clinical illnesses. The extracts are also effective against the gastroenteritis-causing Salmonella typhimurium, which affects both humans and other mammals63.
Insecticidal activity: Heigh level of sinigrin was found in Brassica nigra plants, therefore artificial aphid diet to which sinigrin was selectively added was used to rear the crucifer specialist, Brevicoryne brassicae. Two species of polyphagous ladybird beetle, Adalia bipunctata and Coccinella septempunctata used aphids as food sources. Larval growth of aphids decreased in the presence of sinigrin and increased the time of ladybird larva necessary to reach the second instar. Different numbers of kinds of glucosinolates (GLS) showed increased ladybird larval mortality at higher GLS concentrations with Brassicaceae species. The amount of sinigrin in the diet of B. brassicae indicates that it makes this aphid unsuitable for Adalia bipunctata but not for Coccinella septempunctata64,65. Good chemosuppressive and moderate chemoprophylactic activity were demonstrated by Brassica nigra and the plant may contain biologically active components that are important for the prevention and treatment of malaria66.
Sinigrin was utilized as a fumigation agent against the smaller grain borer, Rhyzopertha dominica and the housefly, Musca domestica. The effectiveness of sinigrin against both species was similar to that of chloropicrin, a fumigant sold for commercial use. The insecticidal properties of glucotropaeolin aglucones were comparable to those of sinigrin aglucones. As fumigants, the gluconate of epiprogoitrin was only marginally effective. For the synthetic analogues of the aglucones sinigrin and a glucotropaeolin, a quantitative structure activity relationship or QSAR, was created67. Brassica seed meal was used to inhibit mosquito larvae and it included one main glucosinolate that, according to HPLC analysis, accounted for more than 98-99% of all glucosinolates68.
Nematicidal activity: The plants of brassicaceae are the most known nematotoxic plants, as the toxicity of their extracts and of GLSs and related hydrolysis products. Several in vitro studies against different phytoparasitic nematode species have been widely investigated69-73. Mustard bran from some mustard species (Brassica Juncea, Brassica campestris and Brassica nigra) is a significant source of sinigrin and AITC (allylisothiocynate) and can be used as an inexpensive, effective and safely handled pesticide or soil fumigants, to control pest such as nematodes74. The application of mustard bran is comparable to that of granular fertilizer. To maximize the handling qualities of the mustard bran, it can be combined with an appropriate agricultural carrier. When the mustard bran comes into touch with water in the soil, myrosinase transforms the sinigrin in the bran into an active pesticide. Additionally, it can be used to make pesticidal aqueous extracts or suspensions that can be sprayed on pests or the soil to control them. Regarding nematicidal activity, mustard aqueous extracts were compared to mustard flour extract, which is known to contain a high quantity of sinigrin. There is great nematicidal activity in mustard bran. Regarding nematicidal activity, mustard aqueous extracts were compared to mustard flour extract, which is known to contain a high quantity of sinigrin. There is great nematicidal activity in mustard bran. The whole mustard seed extract, on the other hand, exhibited very little nematicidal action73. Because mustard bran is a natural and biodegradable product, it is an environmentally friendly insecticide. When converted to AITC, mustard bran, which is an efficient pesticide, can be derived from any variety or species of Brassica that has a high enough husk sinigrin content to provide the needed amount of pest control. A mustard with an effective level of pesticide activity has a sinigrin concentration of about 2.5% by weight74. Myrosinase (MYR, β-thioglucoside glucohydrolase) isoenzymes catalyze the hydrolysis of glucosinolates (GSLs, thioglucosides) found in crop residues, which releases biocidal isothiocyanates (ITCs) that have a particular effect on soilborne diseases75,76. According to in vitro research, exposure to ITCs generated from Brassica plants77 substantially reduces nematode motility, most likely as a result of nematodes’ impaired capacity to identify hosts. In the end, Brassica species may have an impact on the nematode parasitism of potatoes by shrinking the size of the dorsal pharyngeal gland nucleus in Globodera rostochiensis, the potato cystnematode78. A quantitative RT-PCR investigation showed that extracts from brassica leaves can significantly reduce the viability of encysted eggs of Globodera pallida, showing that the biofumigation action can penetrate the cysts’ hard cuticular layer77. Meloidogyne incognita, a root-knot nematode, has similarly been shown to be susceptible to the ovicidal impact of Brassica. Brassicaceae plants showed nematicidal or repellent effect at a high concentration79and were used in this assay because they were previously reported to have toxicity against Meloidogyne incognita when incorporated into soil or used in soil biofumigation80-83. These crops are nematode hosts, but they often control nematode development and infestation84. The Allyl ITCS released from brassicales plant tissue in the field, to act as nematicides have been described, for example defatted seed meals from Brassica nigra, Eruca sativa and Barbarea verna in tomato crops85. Products of Brassica spp. patented for use as biofumigants to control pathogens86-90. It contains severalsecondary metabolites potentially important for an industrial application, glucosinolates are however their most studied constituents91-95. A series of studies addressed the effect of various brassica and mustard cover crops on nematodes in potato systems96.
Molluscicidal activity: Many plant products have shown molluscicidal activity in their different preperations97,98. The toxicity of Brassica nigra seed powder and its different preparations against Lymnaea acuminata. There was a negative regression between the time and LC50 of different preparations of B. nigra against Lymnaea acuminata. Toxicity of column purified fraction of Brassica nigra seed indicates that it has great potential as molluscicides as it kills the snail at a very low concentration (96 hrs LC50-5.96 mg/L) with respect to synthetic as well as plant derived molluscicides99.
CONCLUSION
The present review explores the historical background of black mustard (Brassica nigra) and summarises the research data on the chemical composition, pharmacological properties, cytotoxilogical effects of Brassica nigra. The bioactive compounds of Brassica seeds (proteins, polyphenols, alkaloids, GLS, fatty acids and carotenoids) are responsible for different medicinally significant pharmacological properties such as antioxidant, antimicrobial, anticancerous, insecticidal and nematocidal activities. Further research is needed to investigate the potential of isolated and purified bioactive compounds for use in the food industry and the field of human health.
SIGNIFICANCE STATEMENT
Brassica nigra seeds have been shown to exhibit a variety of biological characteristics, such as anti-inflammatory, antidiabetic, anti-cancer, antibacterial and neuroprotective effects. This has been proven in previous research work. Arundhati Singh initially observed the molluscicidal activity of Brassica nigra seed powder and its various preparations against Lymnaea acuminata. Thus, it may be concluded that Brassica nigra seed powder has molluscicidal efficacy. Brassica nigra seeds are associated with their abundance of active ingredients that help prevent and treat a number of chronic illnesses, includs COVID-19, diabetes, obesity and cancer. Brassica nigra possesses cognitive-enhancing, anti-Parkinson and antimigraine properties. Brassica nigra seeds possess significant anxiolytic, anthelmintic, anticancer and thrombolytic properties which may be a good candidate for treating these diseases through the determination of bio-active lead compounds.
REFERENCES
- Nguyen, T., R. Nandasiri, O. Fadairo and N.A.M. Eskin, 2024. Phenolics of mustard seeds: A review on composition, processing effect and their bioactvities. J. Am. Oil Chem. Soc., 101: 5-21.
- Farell, K.T., 1990. Spices, Condiments and Seasonings. 2nd Edn., Springer, New York, United States, Pages: 414.
- Sambamurthy, A.V.S.S., 2021. Economic Botany of Crop Plants. Asiatech Publishers Inc. New Delhi, India, ISBN: 9788187680031.
- Peter, K.V., 2012. Handbook of Herbs and Spices. 2nd Edn., Woodhead Publishing, Sawston, United Kingdom, ISBN:9780857090393, Pages: 640.
- Amer, W.M., M.A. Shoulkamy, H.D. Abd El-Baset and A.M. Faried, 2022. Infraspecific Identity of the Wild Brassica nigra (L.) Koch. using morphological, cytogenetics and molecular (nuclear and chloroplast) approaches. Jordan J. Biol. Sci., 15: 489-500.
- Tsunoda, S., 1980. Eco-physiology of Wild and Cultivated Forms in Brassica and Allied Genera. In: Brassica Crops and Wild Allies: Biology and Breeding, Tsunoda, S., K. Hinata and C. Gomez-Campo (Eds.), Japan Scientific Societies Press, Tokyo, Japan, pp: 109-120.
- Vaughan, J.G., 1997. A multidisciplinary study of the taxonomy and origin of Brassica crops. BioScience, 27: 35-40.
- Yang, Y.W., P.Y. Tai, Y. Chen and W.H. Li, 2002. A study of the phylogeny of Brassica rapa, B. nigra, Raphanus sativus, and their related genera using noncoding regions of chloroplast DNA. Mol. Phylogenet. E, 23: 268-275.
- Danlami, U., A.T. Orishadipe and D.R. Lawal, 2016. Phytochemical, nutritional and antimicrobial evaluations of the aqueous extract of Brassica nigra (Brassicaceae) seeds. Am. J. Appl. Chem., 4: 161-163.
- Asaad, N.K. and Q.A. Razooqi, 2022. Protective role of the aqueous extract of Brassica nigra seed against cadmium chloride toxicity in lung tissue and hematological parameters of female rats. Mater. Today: Proc., 60: 1497-1501.
- Zafar, H., T. Aziz, B. Khan, Abdul Mannan, Riaz ur Rehman and M. Zia, 2020. CuO and ZnO nanoparticle application in synthetic soil modulates morphology, nutritional contents, and metal analysis of Brassica nigra. ACS Omega, 5: 13566-13577.
- van Ginneken, L., E. Meers, R. Guisson, A. Ruttens and K. Elst et al., 2007. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landscape Manage., 15: 227-236.
- Angelova, V. and K. Ivanov, 2008. Bio-accumulation and distribution of heavy metals in black mustard (Brassica nigra Koch). Environ. Monit. Assess., 153: 449-459.
- Lietzow, J., 2021. Biologically active compounds in mustard seeds: A toxicological perspective. Foods, 10.
- Mazumder, A., A. Dwivedi and J. du Plessis, 2016. Sinigrin and its therapeutic benefits. Molecules, 21.
- Mitreiter, S. and T. Gigolashvili, 2021. Regulation of glucosinolate biosynthesis. J. Exp. Bot., 72: 70-91.
- Stepien, A., K. Wojtkowiak and R. Pietrzak-Fiecko, 2017. Nutrient content, fat yield and fatty acid profile of winter rapeseed (Brassica napus L.) grown under different agricultural production systems. Chilean J. Agric. Res., 77: 266-272.
- Ayadi, J., M. Debouba, R. Rahmani and J. Bouajila, 2022. Brassica genus seeds: A review on phytochemical screening and pharmacological properties. Molecules, 27.
- Ogidi, O.I., O. Omu and P.A. Ezeagba, 2019. Ethno pharmacologically active components of Brassica juncea (brown mustard) seeds. Int. J. Pharm. Res. Dev. 1: 9-13.
- Sontakke, K.S. and S.L. Shinde, 2020. Evaluation of the phytochemical potential of Brassica junceal. Seeds. Vidyabharati Int. Interdiscip. Res. J., 10: 25-29.
- Ekor, M., 2014. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol., 4.
- Saad, B., H. Azaizeh and O. Said, 2005. Tradition and perspectives of arab herbal medicine: A review. Evidence-Based Complementary Altern. Med., 2: 475-479.
- Shrivastava, V., R.S. Tomar, R.K. Mishra, A. Jyoti and S. Kaushik, 2014. Medicinal potential of some mythologically important plants of India: A Review. Int. J. Multidisciplin. Curr. Res., 2: 99-103.
- Dhama, K., K. Karthik, R. Khandia, A. Munjal and R. Tiwari et al., 2018. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-Current knowledge and future prospects. Curr. Drug Metab., 19: 236-263.
- Srivastava, A.K., A. Singh and V.K. Singh, 2023. Ethnomedicinal potential of Indian coffee plum: Flacourtia jangomas. Adv. Biol. Res., 4: 31-36.
- Sonu, A.K.S., V.K. Singh and A. Singh, 2024. Comprehensive review of Adansonia digitata (Kalpvriksha): Ethnobotany, phytochemistry, and potential applications. Algerian J. Bioscie., 5: 13-23.
- Grieve, M., 1984. A Modern Herbal: The Medicinal, Culinary, Cosmetic and Economic Properties, Cultivation and Folklore of Herbs, Grasses, Fungi, Shrubs and Trees with All Their Modern Scientific Uses. Savvas, Midland Ave, United States.
- Uphof, J.C.T., 1959. Dictionary of Economic Plants. J. Cramer, Weinheim, Germany, Pages: 400.
- Weiner, M.A., 1980. Earth Medicine-Earth Food: Plant Remedies, Drugs, and Natural Foods of the North American Indians. Macmillan Publishing Company, Broadway, United States, ISBN: 9780026256100, Pages: 230.
- Agrawal, S., T. Yallatikar and P. Gurjar, 2019. Brassica nigra: Ethnopharmacological review of a routinely used condiment. Curr. Drug Dis. Technol., 16: 40-47.
- Foster, S. and J.A. Duke, 2000. A Field Guide to Medicinal Plants and Herbs of Eastern and Central North America. Houghton Mifflin Harcourt, New York, ISBN: 9780395988145, Pages: 411.
- Hwang, K.A., H.J. Hwang, Y.J. Hwang and Y.J. Kim, 2020. Mustard leaf extract suppresses psychological stress in chronic restraint stress-subjected mice by regulation of stress hormone, neurotransmitters, and apoptosis. Nutrients, 12.
- Ahmed, A.G., U.K. Hussein, A.E. Ahmed, K.M. Kim and H.M. Mahmoud et al., 2020. Mustard seed (Brassica nigra) extract exhibits antiproliferative effect against human lung cancer cells through differential regulation of apoptosis, cell cycle, migration, and invasion. Molecules, 25.
- Fahey, J.W., Y. Zhang and P. Talalay, 1997. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U.S.A., 94: 10367-10372.
- Clarke, J.D., A. Hsu, Z. Yu, R.H. Dashwood and E. Ho, 2011. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res., 55: 999-1009.
- Clarke, J., Z. Yu and E. Ho, 2010. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal and prostate cancer cells. FASEB J., 24: 107.5-107.5.
- Fahey, J.W., S.L. Wehage, W.D. Holtzclaw, T.W. Kensler, P.A. Egner, T.A. Shapiro and P. Talalay, 2012. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res., 5: 603-611.
- Fahey, J.W. and P. Talalay, 1999. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol., 37: 973-979.
- Shapiro, T.A., J.W. Fahey, K.L. Wade, K.K. Stephenson and P. Talalay, 2001. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev., 10: 501-508.
- Yanaka, A., J.W. Fahey, A. Fukumoto, M. Nakayama and S. Inoue et al., 2009. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-Infected mice and humans. Cancer Prev. Res., 2: 353-360.
- Higdon, J.V., B. Delage, D.E. Williams and R.H. Dashwood, 2007. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res., 55: 224-236.
- Fowke, J.H., 2007. Head and neck cancer: A case for inhibition by isothiocyanates and indoles from cruciferous vegetables. Eur. J. Cancer Prev., 16: 348-356.
- Juge, N., R.F. Mithen and M. Traka, 2007. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci., 64: 1105-1127.
- Zhao, H., J. Lin, H.B. Grossman, L.M. Hernandez, C.P. Dinney and X. Wu, 2007. Dietary isothiocyanates, GSTM1, GSTT1, NAT2 polymorphisms and bladder cancer risk. Int. J. Cancer, 120: 2208-2213.
- Boscaro, V., L. Boffa, A. Binello, G. Amisano, S. Fornasero, G. Cravotto and M. Gallicchio, 2018. Antiproliferative, proapoptotic, antioxidant and antimicrobial effects of Sinapis nigra L. and Sinapis alba L. extracts. Molecules, 23.
- Ahmed, S.A. and E.M. Kamel, 2013. Chemical constituents, cytotoxic and antibacterial activities of the aerial parts of Brassica nigra. Int. J. Bioassays, 2: 1134-1138.
- Supraja, G., K.C. Maheswari, D. Pamarthy and K.V.R. Saritha, 2022. Phytochemicals for Hepatocellular Carcinoma Therapy: From In vitro to Clinic. In: Theranostics and Precision Medicine for the Management of Hepatocellular Carcinoma, Volume 3: Translational and Clinical Outcomes, Nagaraju, G.P. and S. Ahmad (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-323-99283-1, pp: 109-132.
- Uddin, M.S., M.S. Millat, M.S. Islam, M.S. Hussain, M.G. Uddin, S.A. Siddiqui and M. Ferdous, 2020. Exploration of in vitro thrombolytic, anthelminthic, cytotoxic and in vivo anxiolytic potentials with phytochemical screening of flowers of Brassica nigra. Future J. Pharm. Sci., 6.
- Regitha, R.N., V. Parthasarathy and N. Balakrishnan, 2021. Cancer protective effect of Brassicca nigra and role of its chemical constituents. Res. J. Pharm. Tech., 14: 1115-1121.
- Salman, I.N., D.B. Hanna and B. Abdul-Razzaq Mshimesh, 2022. Antiproliferative activity of Brassica nigra seeds extract in liver tissue of mice exposed to phenobarbital. Al Mustansiriyah J. Pharm. Sci., 22: 8-22.
- da Silva Mattosinhos, P., M.M. Sarandy, R.D. Novaes, D. Esposito and R.V. Gonçalves, 2022. Anti-inflammatory, antioxidant, and skin regenerative potential of secondary metabolites from plants of the Brassicaceae family: A systematic review of in vitro and in vivo preclinical evidence (biological activities Brassicaceae skin diseases). Antioxidants, 11.
- Badrul Alam, M.S. Hossain and M.E. Haque, 2011. Antioxidant and anti-inflammatory activities of the leaf extract of Brassica nigra. Int. J. Pharm. Sci. Res., 2: 303-310.
- Rajamurugan, R., A. Suyavaran, N. Selvaganabathy, C.H. Ramamurthy, G.P. Reddy, V. Sujatha and C. Thirunavukkarasu, 2012. Brassica nigra plays a remedy role in hepatic and renal damage. Pharm. Biol., 50: 1488-1497.
- de Saravia, S.G.G. and C.C. Gaylarde, 1998. The antimicrobial activity of an aqueous extract of Brassica negra. Int. Biodeterior. Biodegrad., 41: 145-148.
- Mejía-Garibay, B., E. Palou and A.L. Pez-Malo, 2015. Composition, diffusion, and antifungal activity of black mustard (Brassica nigra) essential oil when applied by direct addition or vapor phase contact. J. Food Protec., 78: 843-848.
- Aguilar-González, A.E., E. Palou and A. López-Malo, 2015. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovative Food Sci. Emerging Technol., 32: 181-185.
- Olivier, C., S.F. Vaughn, E.S.G. Mizubuti and R. Loria, 1999. Variation in allyl isothiocyanate production within Brassica species and correlation with fungicidal activity. J. Chem. Ecol., 25: 2687-2701.
- Reyes-Jurado, F., T. Cervantes-Rincón, H. Bach, A. López-Malo and E. Palou, 2019. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod., 131: 90-95.
- Nanetti, A., L. Ugolini, G. Cilia, E. Pagnotta and L. Malaguti et al., 2021. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms. 9.
- Bhatia, M. and A. Sharma, 2024. Evaluation of few bioactive components of spice origin for their antimicrobial potential towards microbes commonly implicated in food spoilage and foodborne pathogenesis. J. Appl. Nat. Sci., 16: 308-314.
- Senanayake, D., P.J. Torley, J. Chandrapala and N.S. Terefe, 2023. Microbial fermentation for improving the sensory, nutritional and functional attributes of legumes. Fermentation, 9.
- Jasim, A.S., 2012. Antibacterial activity of oils extracts of Brassica nigra seeds on some bacteria isolated from plaque and healthy teeth in children (1-5) years. Basrah J. Sci., 30: 105-119.
- Farhat Ullah, M. Ayaz, Abdul Sadiq, Farman Ullah and I. Hussain et al., 2020. Potential role of plant extracts and phytochemicals against foodborne pathogens. Appl. Sci., 10.
- Francis, F., G. Lognay, J.P. Wathelet and E. Haubruge, 2001. Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. J. Chem. Ecol., 27: 243-256.
- Pratt, C., T.W. Pope, G. Powell and J.T. Rossiter, 2008. Accumulation of glucosinolates by the cabbage aphid Brevicoryne brassicae as a defense against two Coccinellid species. J. Chem. Ecol., 34: 323-329.
- Muluye, A.B., E. Melese and G.M. Adinew, 2015. Antimalarial activity of 80 % methanolic extract of Brassica nigra (L.) Koch. (Brassicaceae) seeds against Plasmodium berghei infection in mice. BMC Complementary Altern. Med., 15.
- Peterson, C.J., R. Tsao and J.R. Coats, 1998. Glucosinolate aglucones and analogues: Insecticidal Insecticidal properties and a QSAR. Pestic. Sci., 54: 35-42.
- Flor-Weiler, L.B., R.W. Behle, M.A. Berhow, S.P. McCormick, S.F. Vaughn, E.J. Muturi and W.T. Hay, 2023. Bioactivity of brassica seed meals and its compounds as ecofriendly larvicides against mosquitoes. Sci. Rep., 13.
- Lazzeri, L., G. Curto, E. Dallavalle, L. D'Avino, L. Malaguti, R. Santi and G. Patalano, 2009. Nematicidal efficacy of biofumigation by defatted brassicaceae meal for control of Meloidogyne incognita (Kofoid et White) Chitw. on a full field zucchini crop. J. Sustainable Agric., 33: 349-358.
- Eugui, D., C. Escobar, P. Velasco and J. Poveda, 2022. Glucosinolates as an effective tool in plant-parasitic nematodes control: Exploiting natural plant defenses. Appl. Soil Ecol., 176.
- Avato, P., T. D'Addabbo, P. Leonetti and M.P. Argentieri, 2013. Nematicidal potential of Brassicaceae. Phytochem. Rev., 12: 791-802.
- Wu, H., C.J. Wang, X.W. Bian, S.Y. Zeng and K.C. Lin et al., 2011. Nematicidal efficacy of isothiocyanates against root-knot nematode Meloidogyne javanica in cucumber. Crop Prot., 30: 33-37.
- Oliveira, R.D.L., O.D. Dhingra, A.O. Lima, G.N. Jham, M.A. Berhow, R.K. Holloway and S.F. Vaughn, 2011. Glucosinolate content and nematicidal activity of Brazilian wild mustard tissues against Meloidogyne incognita in tomato. Plant Soil, 341: 155-164.
- Tsao, R., Q. Yu, I. Friesen, J. Potter and M. Chiba, 2002. Factors affecting the dissolution and degradation of oriental mustard-derived sinigrin and allyl isothiocyanate in aqueous media. J. Agric. Food Chem., 48: 1898-1902.
- Matthiessen, J.N. and J.A. Kirkegaard, 2006. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci., 25: 235-265.
- Motisi, N., T. Doré, P. Lucas and F. Montfort, 2010. Dealing with the variability in biofumigation efficacy through an epidemiological framework. Soil Biol. Biochem., 42: 2044-2057.
- Lord, J.S., L. Lazzeri, H.J. Atkinson and P.E. Urwin, 2011. Biofumigation for control of potato cyst nematodes: Activity of brassica leaf extracts and green manures on Globodera pallida in vitro and in soil. J. Agric. Food Chem., 59: 7882-7890.
- Bhatta, B., L.M. Dandurand, M.J. Morra and I. Popova, 2023. Control of Globodera pallida using Brassica juncea seed meal extract and the trap crop Solanum sisymbriifolium. Plant Dis., 107: 1809-1815.
- Golec, A.F.C., 2018. In vitro study on the nematicidal effect of different plant extracts on Pratylenchus penetrans and Meloidogyne chitwoodi., Rev. Facultad Nacional de Agronomía Medellín, 72: 8945-8952.
- Javed, N., S.R. Gowen, M. Inam-ul-Haq and S.A. Anwar, 2007. Protective and curative effect of neem ( Azadirachta indica) formulations on the development of root-knot nematode Meloidogyne javanica in roots of tomato plants. Crop Protect., 26: 530-534.
- Patil, J.A., A. Kumar, S. Yadav and K.K. Verma, 2020. Nematicidal effect of cruciferous bio-fumigants against the root-knot nematode, Meloidogyne incognita infesting okra. J. Nematol., 52: 1-7.
- Monfort, W.S., A.S. Csinos, J. Desaeger, K. Seebold, T.M. Webster and J.C. Diaz-Perez, 2007. Evaluating Brassica species as an alternative control measure for root-knot nematode (M. incognita) in Georgia vegetable plasticulture. Crop Prot., 26: 1359-1368.
- Barros, A.F., V.P. Campos, J.C.P. da Silva, M.P. Pedroso, F.H.V. Medeiros, E.A. Pozza and A.L. Reale, 2014. Nematicidal activity of volatile organic compounds emitted by Brassica juncea, Azadirachta indica, Canavalia ensiformis, Mucuna pruriens and Cajanus cajan against Meloidogyne incognita. Appl. Soil Ecol., 80: 34-43.
- McLeod, R.W., J.A. Kirkegaard and C.C. Steel, 2001. Invasion, development, growth and egg laying by Meloidogyne javanica in Brassicaceae crops. Nematology, 3: 463-472.
- Curto, G., E. Dallavalle, R. Matteo and L. Lazzeri, 2016. Biofumigant effect of new defatted seed meals against the southern root-knot nematode, Meloidogyne incognita. Ann. Appl. Biol., 169: 17-26.
- Lazzeri, L., L. Malaguti, S. Cinti, L. Ugolini and G.R. de Nicola et al., 2013. The Brassicaceae biofumigation system for plant cultivation and defence. An Italian twenty-year experience of study and application. Acta Hortic., 1005: 375-382.
- de Nicola, G.R., L. D'Avino, G. Curto, L. Malaguti and L. Ugolini et al., 2013. A new biobased liquid formulation with biofumigant and fertilising properties for drip irrigation distribution. Ind. Crops Prod., 42: 113-118.
- Ladhalakshmi, D., R. Madhubala, S. Sundravadana, G.S. Laha, D. Krishnaveni, G. Sangeetha and G. Ragothuman, 2015. Biofumigation in Crop Disease Management. In: Sustainable Crop Disease Management Using Natural Products, Ganesan, S.G.S., K.V.K. Vadivel and J.J.J. Jayaraman (Eds.), CABI International, Wallingford, United Kingdom, ISBN: 978-1-78064-323-6, pp: 389-402.
- Mossa, M.I., 2021. Effect of biofumigation with selected Brassicaceae plants on the count and diversity of soil-borne fungi in Arish City, Egypt. CATRINA, 24: 81-88.
- Dutta, T.K., M.R. Khan and V. Phani, 2019. Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: Current status and future prospects. Curr. Plant Biol., 17: 17-32.
- Daxenbichler, M.E., C.H. VanEtten and P.H. Williams, 1979. Glucosinolates and derived products in cruciferous vegetables. Analysis of 14 varieties of Chinese cabbage. J. Agric. Food Chem., 27: 34-37.
- Daxenbichler, M.E., G.F. Spencer, D.G. Carlson, G.B. Rose, A.M. Brinker and R.G. Powell, 1991. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry, 30: 2623-2638.
- Mithen, R., 2001. Glucosinolates-biochemistry, genetics and biological activity. Plant Growth Regul., 34: 91-103.
- Mithen, R., R. Bennett and J. Marquez, 2010. Glucosinolate biochemical diversity and innovation in the Brassicales. Phytochemistry, 71: 2074-2086.
- Branca, F., G. Li, S. Goyal and C.F. Quiros, 2002. Survey of aliphatic glucosinolates in Sicilian wild and cultivated Brassicaceae. Phytochemistry, 59: 717-724.
- Larkin, R.P. and R.P. Lynch, 2018. Use and effects of different Brassica and other rotation crops on soilborne diseases and yield of potato. Horticulturae, 4.
- Singh, A. and V.K. Singh, 2009. Molluscicidal activity of Saraca asoca and Thuja orientalis against the fresh water snail Lymnaea acuminata. Vet. Parasitol., 164: 206-210.
- Singh, N.V., A. Singh and V.K. Singh, 2024. Laboratory assessment of molluscicidal activities of Cannabis sativa, Acacia nilotica, and Tinospora cordifolia against snail host of Fasciola spp. Vector-Borne Zoonotic Dis., 24: 382-389.
- Singh, K., A. Singh and D.K. Singh, 1996. Molluscicidal activity of neem (Azadirachta indica A.Juss). J. Ethnopharmacol., 52: 35-40.
How to Cite this paper?
APA-7 Style
Singh,
A., Singh,
V.K. (2025). Chemical and Pharmacological Activities of Rape Seed Mustard Brassica nigra (Black Mustard): A Magic Herb. Asian Journal of Biological Sciences, 18(1), 43-54. https://doi.org/10.3923/ajbs.2025.43.54
ACS Style
Singh,
A.; Singh,
V.K. Chemical and Pharmacological Activities of Rape Seed Mustard Brassica nigra (Black Mustard): A Magic Herb. Asian J. Biol. Sci 2025, 18, 43-54. https://doi.org/10.3923/ajbs.2025.43.54
AMA Style
Singh
A, Singh
VK. Chemical and Pharmacological Activities of Rape Seed Mustard Brassica nigra (Black Mustard): A Magic Herb. Asian Journal of Biological Sciences. 2025; 18(1): 43-54. https://doi.org/10.3923/ajbs.2025.43.54
Chicago/Turabian Style
Singh, Arundhati, and Vinay Kumar Singh.
2025. "Chemical and Pharmacological Activities of Rape Seed Mustard Brassica nigra (Black Mustard): A Magic Herb" Asian Journal of Biological Sciences 18, no. 1: 43-54. https://doi.org/10.3923/ajbs.2025.43.54

This work is licensed under a Creative Commons Attribution 4.0 International License.



