NO/cGMP/KATP Pathway and PPAR Receptors Contribution in MLR-1023 Neuroprotection in LPS-Induced Mice Model of AD
| Received 19 Jul, 2024 |
Accepted 05 Sep, 2024 |
Published 31 Dec, 2024 |
Background and Objective: The occurrence of neurodegenerative disease has increased because of relation between diabetes and Alzheimer’s disease. In this study, the effects of MLR-1023 (Tolimidone) on depression, hyperalgesia and hippocampal TNF-α level were studied in a model of Alzheimer’s disorder, produced by Lipopolysaccharide (LPS) injection. The role of PPARγ receptors and NO/cGMP/KATP-channels pathway was examined for determining likely engaged mechanisms. Materials and Methods: The AD mice model was made by LPS. The first stage was designed to find the effective dose of MLR-1023. In this stage, MLR-1023 (20, 30 and 40 mg/kg/i.p.) was given to treatment groups. The second stage is to show the potential mechanisms of mice pre-treated with their antagonist/agonist. Behavioral assay was performed including; Open-field assay, forced-swimming behavior and hot-plate exam. Then the mice hippocampus was removed and TNF-α levels were measured. Results: The LPS increased FST immobility but MLR-1023 reduced it. Methylene blue, L-NAME and glibenclamide intensified it. The L-arginine, sildenafil and diazoxide weakened it. The LPS reduced pain threshold, while increased by MLR-1023. The L-NAME, methylene blue, glibenclamide and GW9662 reduced it. It was increased by L-arginine, diazoxide and pioglitazone. The MLR-1023 decreased TNF-α. Methylene blue, L-NAME, glibenclamide and GW9662 increased that. It was decreased by L-arginine, sildenafil and pioglitazone. Conclusion: The MLR-1023 can improve depression, hyperalgesia and neuroinflammation induced by LPS in mice models and NO/cGMP/KATP-channels signaling pathway and PPARγ receptors play a probable role in this effect.
| Copyright © 2024 Dolatshahi et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Type 2 diabetes has a notable relation with Alzheimer’s disease (AD), but its mechanisms are unclear based on several evidences1. So, several investigations were done to introduce possible neuroprotective effects of diabetic drugs. However, more focus is required2. Many evidences justify a significant connection between neuroinflammation and AD pathology but mechanisms may be still unclear3. Besides, neuroinflammation is an important difficulty in diabetic patients, but hasn’t been introduced any specific drug up to now, though4.
Depression is a common symptom of AD and mutually can also be a remarkable risk factor for it. Hence, stress-related ailments may be involved in both. In both pathologies, it is notable that clinical observations indicate atrophies in the same brain regions such as hippocampus5. The brain’s emotionally controlling parts damage can cause depression, in AD. This depression is severe in early AD and could be a psychosomatic response. But, in the late stages, the cognitive impairments reduce the responsive expressions6. Some evidences indicate many changes in opioid system in animal models as an available mechanism in altered pain experiences in AD, suggesting new approach of opioid analgesic use in AD7. Glial cells show a basic role in the chronic pain signaling modulation and sensitization of the nervous system by pro-inflammatory mediator’s required production2. So, it convinced us to focus on pain threshold and depressive-like performance in the mouse AD model.
The MLR-1023 (Tolimidone) is a notable allosteric Lyn-kinase agonist that can decrease blood sugar, rapid-onset and long-lasting. So, it means that Lyn-kinase agonists might be a different way to manage diabetes. This insulin receptor-involvement of Lyn kinase suggests that MLR-1023 controls glucose levels by insulin-signaling modulation8. The Lyn kinase targeting drugs suggest a new therapeutic way to improve insulin signaling without a change in insulin secretion level. Also, it can decrease blood glucose which can be helpful in neurodegenerative disorders such as AD.
The purpose of the current project was to find out the consequences of MLR-1023 treatment on depression, hyperalgesia and brain TNF-α levels in Lipopolysaccharide (LPS) induced mice model of AD. The probable function of PPAR-gamma receptors and NO/cGMP/KATP channels pathway were examined to define the probable mechanisms.
MATERIALS AND METHODS
The study was carried out from April, 2021 to March, 2022.
Mice: Ninety male NMRI mice (weighing 25-30 g) were bought from AJUMS (Ahvaz University of Medical Sciences, Iran). They had free food and water access and a 12 hrs light-dark set at a 22±2°C temperature and 80% humidity. This study is approved under the ethical approval code of DUMS (Dezful University of Medical Sciences, Iran), IR.DUMS.REC.1399.043. Each task has been done based on the NIH Guide for the Care and Use of Laboratory Animals. The mice number was reduced to six mice, per group.
Drugs: Some medications were purchased from Sigma Aldrich (USA) included: an ATP sensitive K+ channel blocker (Glibenclamide), an ATP sensitive K+ channel opener (diazoxide), a PDE-5 blocker (sildenafil), a NO precursor (L-arginine), a non-specific NOS blocker [L-NAME, N(G)-nitro-L-argininemethyl ester], Lipopolysaccharide (LPS), Tolimidone [MLR-1023; CP-26154; 2(1H)-pyrimidinone, 5-(3-methylphenoxy)]. The PPARγ-agonist (pioglitazone) was obtained from Osveh Co (Iran). Also, a PPARγ-antagonist (GW9662) was purchased from Tocris Bioscience (Bristol, UK). A guanylyl cyclase/NOS blocker (Methylene blue) was obtained from Merck (Germany). The MLR-1023, GW9662, pioglitazone and glibenclamide were freshly dissolved in 1% DMSO (dimethyl sulfoxide) (obtained from Fermentas Life Sciences, Lithuania). Then diluted up to 10 times the primary volume with saline. Other medications were diluted in normal saline. All drugs were injected i.p. (intraperitoneally, 10 mL/kg). The medication protocol (way, timing and doses) was conducted according to previous reports9,10.
Behavioral assay: The behavioral assay was performed by tests including; forced-swimming, open-field and hot-plate. Next, the mice brains were removed and to evaluate the inflammatory status, the amount of inflammatory factor TNF-α in hippocampal tissue samples was measured.
Open field trial (OFT): To confirm that variations in the mice’s locomotion did not have a key role in the other behavioral results, the mice’s locomotion was tested by open field trial immediately before the other behavioral tests. The tool (Borj Sanat Co. Tehran, Iran) has a wooden box (40×60×50 cm). The bottom of the device was divided equally (12 squares). The mice were individually put in the left corner of the field and could move freely. In 6 min, the square number crossing with all paws was recorded. The trial environment was in dim light condition, minimizing mouse stress. At the end of each test, the trial tool’s environment was washed with ethanol (10% solution), minimizing mouse residuals9.
Forced-swimming trial (FST): For evaluating the depressive-like behavior, the FST was done. Every mouse was released in a cylindrical water tank (Borj Sanat Co. Tehran, Iran) with 10 cm diameter and 25 cm height. It had 19 cm of water (height) at 25±1°C temperature. The mice could swim freely for 6 min. Then, the motionlessness duration in the last 4 min of the test was recorded. When the mouse stopped struggling and remained immobile and did only essential movements to hold his head out of the water, it was considered as immobility time9.
Hot-plate trial: The device consists of a Plexiglas cylinder located on a hot metal plate (52±0.2°C, Borj Sanat Co. Tehran, Iran). Pain threshold is considered as duration between mouse release on plate to observation of first response such as; jumping out of the cylinder or licking the back foot. Every mouse had 2 min adaptation time with device, day before trial day. Every mouse was released on the hot plate and the response latency was noted. The cut-off time (40 sec) is considered to prevent tissue injury. At the end of each test, the trial tool’s environment was washed with a 10% ethanol solution11.
Measurement of brain TNF-α: The animals were beheaded, after deep anesthesia. The hippocampus as soon as possible was extracted, washed with saline and moved to -80°C freezer. On the day of the cytokines level assay, every sample was weighed, homogenized, shaken (90 min) and centrifuged (at 4°C, 4000×g, for 15 min). After that, the supernatant was picked up12. ELISA kit for TNF-α was purchased from the LDN Immunoassays Company (Germany) and the test protocol was carried out based on the kit’s guide.
Treatments: This model was made by LPS injection. On the first day, every mouse received LPS (0.25 mg/kg, 0.1 mL/10 g of weight, 7 days). Except for the control group that was injected similar volume of vehicle (normal saline) instead13,14. The study was designed in two stages; the first stage was designed to find the effective dose of MLR-1023 on behavioral assays. In this stage, 30 min before the tests, MLR-1023 (20, 30 and 40 mg/kg/i.p. or 10 mL/kg vehicle) was given to treatment groups for 14 consecutive days. In the second stage; to show the potential role of the NO in the MLR-1023 results, mice pre-treated with their antagonist/agonist; L-arginine (750 mg/kg, i.p.)/L-NAME (10 mg/kg, i.p.), respectively or vehicle 30 min before MLR-1023 (40 mg/kg, i.p.)15. Similarly, cyclic GMP involvement was evaluated by pre-treatment with methylene blue (20 mg/kg, i.p.)/sildenafil (5 mg/kg, i.p.) or vehicle before MLR-102316. Also, regarding the role of KATP channel gating, pre-treatment with glibenclamide (1 mg/kg, i.p.)/diazoxide (5 mg/kg, i.p.) or vehicles 30 min before MLR-1023 were used17. Finally, for designating the possible role of PPARγ receptors pre-treatment with their agonist/antagonist (pioglitazone (5 mg/kg, i.p.)/ GW9662 (2 mg/kg, i.p.)) or vehicles were injected 30 min before MLR-1023 (40 mg/kg, i.p.). Medication doses were selected from previous report of Shahsavarian et al.15. About 30 min after MLR-1023 treatment, the behavioral assays were done.
Statistical analysis: The statistical analysis was carried out using SPSS software (version 22). The data was shown by Mean±SEM. All data had normal distribution (by Kolmogorov-Smirnov test). One-way ANOVA (followed by Tukey’s post hoc test, or LSD in some cases) was used for group comparisons. A significant difference is defined as; p<0.05.
RESULTS
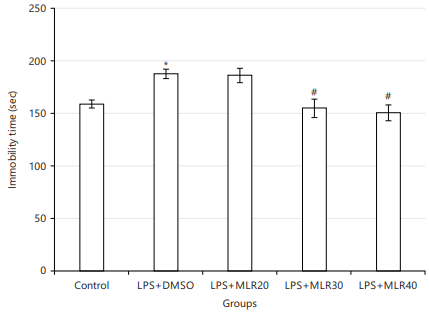
Effect of MLR-1023 on locomotor activity and forced-swimming test (FST): No significant differences were seen among all of the groups in open-field test (p>0.05). Lipopolysaccharide (LPS) injection significantly increased the motionlessness time versus control group (*p<0.05; Fig. 1) in the forced-swimming test. Anti-immobility effect of MLR-1023 (20, 30 and 40 mg/kg), was shown in Fig. 1. The MLR-1023 (30 and 40 mg/kg) showed significant anti-immobility effects (#p<0.05; Fig. 1). In the following, to show the potential role of the NO/cGMP/KATP pathway and PPARγ receptors in MLR-1023 effects, mice received pre-treatment by related agonists and antagonists which is mentioned below. These agonists and antagonist didn’t exert significant effect in FST when used alone.
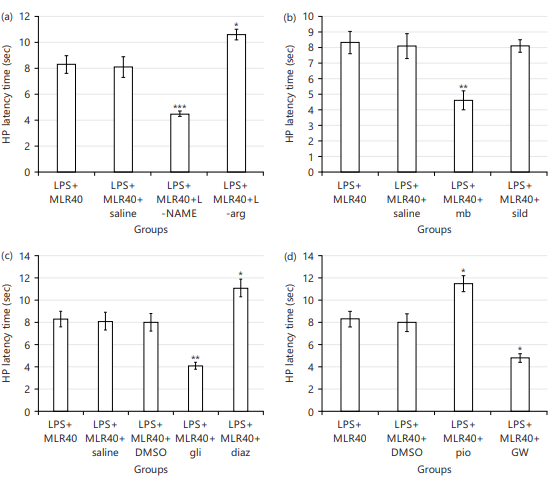
NO involvement in the MLR-1023 antidepressant effect in the forced swimming test: As it has been shown in Fig. 2a, pre-treatment by L-NAME (10 mg/kg) increased (*p<0.05) the antidepressant effect of MLR-1023 (40 mg/kg). While L-arginine pretreatment (750 mg/kg) prevented the antidepressant effect of MLR-1023 (40 mg/kg) in the FST (**p<0.01).
cGMP involvement in the MLR-1023 antidepressant effect in forced swimming test: Methylene blue (20 mg/kg) pre-treatment increased the antidepressant effect of MLR-1023 (*p<0.05) by reducing the immobility time in FST test. While pre-treatment by sildenafil (5 mg/kg) prevented the antidepressant effect of MLR-1023 (*p<0.05) by increasing the immobility time (Fig. 2b).
KATP channels involvement in the MLR-1023 antidepressant effect in forced swimming test: As it shown in Fig. 2c, glibenclamide (1 mg/kg) in combination by MLR-1023 (40 mg/kg) lead to an anti-immobility effect in the FST (*p<0.05). Whereas, diazoxide pre-treatment (10 mg/kg) decreased the MLR-1023 antidepressant effect (**p<0.01) through increasing the motionlessness time.
PPARγ receptors involvement in the MLR-1023 antidepressant effect in forced swimming test: For this, pioglitazone (5 mg/kg) and GW9662 (2 mg/kg) were used in combination with MLR-1023 (40 mg/kg). But, they didn’t significantly change the antidepressant effect of MLR-1023 (Fig. 2d).
|

|
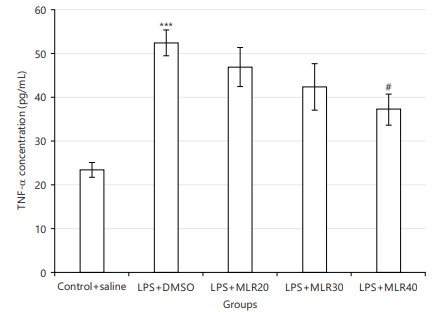
Effects of MLR-1023 on pain threshold: The LPS significantly decreased the pain threshold (HP latency time) compared to control group (*p<0.05; Fig. 3) in hot-plate test. The antihyperalgesic effect of the MLR-1023 (20, 30 and 40 mg/kg), has been shown in Fig. 3. The MLR-1023 increased the pain threshold significantly only at the dose of 40 mg/kg (#p<0.05; Fig. 3). For showing NO/cGMP/KATP pathway and PPARγ receptors contribution in the MLR-1023 effects, mice were pre-treated with related agonists and antagonists. These agonists and antagonist didn’t show significant effect on hot plate latency time, when used alone.
NO contribution in the MLR-1023 antihyperalgesic effect: The L-NAME (10 mg/kg) Pre-treating decreased (***p<0.001) the pain threshold against LPS+MLR-1023 (40 mg/kg) treated group. While pre-treatment by L-arginine (750 mg/kg) increased (*p<0.05) the pain threshold comparing LPS+MLR-1023 (40 mg/kg) received group (Fig. 4a).
cGMP contribution in the MLR-1023 antihyperalgesic effect: For this aim, methylene blue (20 mg/kg) and sildenafil (5 mg/kg) were pre-used in combination with MLR-1023 (40 mg/kg). Methylene blue decreased (**p<0.01) the pain threshold against LPS+MLR-1023 (40 mg/kg) received group. While sildenafil (5 mg/kg) didn’t have a significant effect (Fig. 4b).

|
|
|
KATP channels contribution in the MLR-1023 antihyperalgesic effect: As shown in Fig. 4c, pretreatment with glibenclamide (1 mg/kg) led to decrease (**p<0.01) in the pain threshold compared to LPS+MLR-1023 (40 mg/kg, i.p.) treated group. Whereas pre-treating with diazoxide (10 mg/kg) increased the pain threshold compared to LPS+MLR-1023 (40 mg/kg) received group (*p<0.05).
PPARγ receptors contribution in the MLR-1023 antihyperalgesic effect: Coadministration of pioglitazone (5 mg/kg) increased the pain threshold compared to LPS+MLR-1023 (40 mg/kg, i.p.) received mice (*p<0.05). However, pre-treatment by GW9662 (2 mg/kg) decreased (*p<0.05) the pain threshold compared to LPS+MLR-1023 (40 mg/kg) received mice (Fig. 4d).
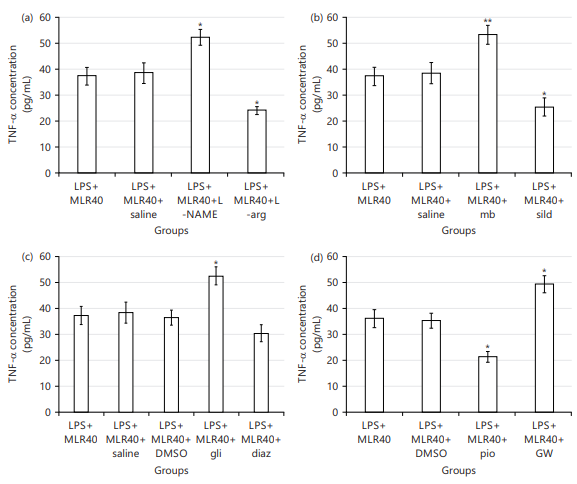
Effect of MLR-1023 on brain tissue inflammation: Brain tissue TNFalpha (TNF-α) levels (pg/mL) was measured as a neuroinflammatory marker, whereas it was increased by LPS usage (Fig. 5) versus control (***p<0.001). The anti-inflammatory effect of MLR-1023 (i.p., 20, 30 and 40 mg/kg), has been displayed in Fig. 5. The MLR-1023 (40 mg/kg) significantly decreased the levels of TNF-α (#p<0.05; Fig. 5). For studying the NO/cGMP/KATP pathway and PPARγ receptors potential roleplay, mice were pre-treated with related agonists and antagonists mentioned below. These agonists and antagonist didn’t show significant effects on the TNF-α levels, when used alone.
NO contribution to the anti-inflammatory effect of MLR-1023: Coadministration of L-NAME (10 mg/kg) with MLR-1023 lead to an increase (*p<0.05) in the TNF-α concentration versus LPS+MLR-1023 (40 mg/kg, i.p.) preserved group. While L-arginine (750 mg/kg) decreased (*p<0.05) the TNF-α concentration comparing LPS+MLR-1023 (40 mg/kg) received group (Fig. 6a).
cGMP contribution in the anti-inflammatory effect of MLR-1023: Coadministration of methylene blue (20 mg/kg) with MLR-1023 increased (**p<0.01) the TNF-α concentration versus LPS+MLR-1023 (40 mg/kg) group. While sildenafil (5 mg/kg) decreased (*p<0.05) the TNF-α concentration versus LPS+MLR-1023 (40 mg/kg) received mice (Fig. 6b).
|
KATP channels contribute to the anti-inflammatory effect of MLR-1023: Glibenclamide (1 mg/kg) led to an increase (*p<0.05) in the TNF-α concentration versus LPS+MLR-1023 (40 mg/kg, i.p.) group. Whereas, diazoxide (10 mg/kg) didn’t show any significant result (Fig. 6c).
PPARγ receptors contribution to the anti-inflammatory effect of MLR-1023: Pre-treating by pioglitazone (5 mg/kg) decreased the TNF-α quantities versus LPS+MLR-1023 (40 mg/kg) received mice (*p<0.05). But, GW9662 (2 mg/kg) increased (*p<0.05) the TNF-α concentration comparing LPS+MLR-1023 (40 mg/kg) received mice (Fig. 6d).
DISCUSSION
The results show that LPS injection increased the immovability period in forced swimming tests which means LPS has induced a kind of depression-like behavior. Lipopolysaccharides have been using commonly to make an animal model for depression-like behavior. The LPS can stimulate mice’s immune system, causing symptoms such as weight loss, reduced food and water intake and locomotion18,19. While, the prescription of MLR-1023 reduced this LPS-induced immovability period, indicating an anti-depressant-like effect of MLR-1023. So, it can be inferred MLR-1023 can improve LPS-induced depression-like behavior.
The NO/cGMP pathway can exert a roleplay in many neurophysiological and pathophysiological instances such as neurodegenerative diseases and depression20. Similarly, the current data indicated that co-administration of L-NAME increased the anti-depressive effect of MLR-1023. While pre-treatment with L-arginine (NOS substrate) avoided the anti-depressive effect of MLR-1023. So, NO can reduce the MLR-1023 anti-depressive effect. Hence, NO pathway has a contribution to the MLR-1023 anti-depressive effect. Moreover, since there was no significant change in the locomotion test, it can be concluded that these effects are anti-depressive effects.
Methylene blue co-administration (NOS and sGC inhibitor) increased the anti-depressive effect of MLR-1023 by reducing the immovability period in FST. While sildenafil (as a selective PDE5 inhibitor) prevented the antidepressant-like effect of MLR-1023 by increasing that, indicating an inhibitory effect of cGMP on the MLR-1023 anti-depressive effect. This evidence confirmed cGMP’s contribution to the MLR-1023 anti-depressive effect. Thus, this effect may be mediated through the reduction of cGMP, due to NO synthesis decrease. So, NO/cGMP pathway can play a key role in this antidepressant-like effect20. Glibenclamide (a KATP channel blocker) in combination with MLR-1023 leads to an anti-immobility effect in the FST. So, it increased the MLR-1023 anti-depressive effect. Whereas diazoxide (a KATP channel opener) decreased the MLR-1023 anti-depressive effect by increasing the immobility time in the FST. It shows an inhibitory effect of KATP channels gating on the MLR-1023 anti-depressive effect. So, blockage of NO/cGMP signaling can lead to blocking these channels and exert an anti-depressive effect21.
The PPARγ receptors exist in various brain regions that play a key role in depression, suggesting these receptors contribute to depression as far as involvement in anti-depressive effects of some drugs in FST20. Despite this evidence, the present results showed that pre-treatment with pioglitazone and GW9662 didn’t change the anti-depressive effect of MLR-1023. So, the contribution of PPARγ receptors in the MLR-1023 anti-depressive effect cannot be inferred here. Since LPS can produce inflammatory cytokines and sensitization of nociceptors, it has been commonly used to induce inflammatory pain in animal models22. Similarly, results show that LPS reduced the nociception threshold (HP latency time). So, this study stated that LPS induced a kind of hyperalgesia in mice. Moreover, treatment with MLR-1023 raised the pain threshold, indicating an anti-hyperalgesic effect that improved the hyperalgesia induced by LPS.
Based on previous reports NO/cGMP path contributes to nociception signaling. For example, hydrogen sulfide may attenuate diabetic neuropathic pain through this path23. This pathway exerts nociceptive and anti-nociceptive effects by downstream nociception mediators24. Likewise, in the present study L-NAME reduced the nociception threshold while increasing through L-arginine. These effects confirm the Involvement of NO in the MLR-1023 antihyperalgesic effect. By the way, several studies have shown that NO/cGMP/KATP pathway has a key role in the drug-induced anti-nociception and anti-inflammatory effects25. Here, Methylene blue reduced the nociception threshold. Therefore, it is concluded that maybe cGMP as well as NO raises the nociception threshold and through this way contributes to the MLR-1023 anti-hyperalgesic effect.
Also, it has been shown, NO/cGMP pathway stimulation may lead to potassium channels opening and produce anti-nociceptive effects26. In current study, glibenclamide reduced the nociception threshold and diazoxide increased the nociception threshold. So, the opening of KATP channels may have a possible contribution to the MLR-1023 anti-hyperalgesic effect.
Studies have shown, that the overexpression of PPARγ receptors can create both anti-nociception and anti-inflammatory effects by inhibiting some signaling pathways in spinal microglia27. In one case, Rosiglitazone (a PPARγ receptor agonist) weakened neuropathic pain through activating of PPARγ, which was reversed by GW966228. Similarly, in the present study pre-treatment with pioglitazone (a PPARγ receptor agonist) increased the pain threshold. But pre-treatment with GW9662 (a PPARγ receptor antagonist) decreased the pain threshold. Collectively, it can be concluded that maybe PPARγ receptors are involved in the MLR-1023 anti-hyperalgesia effect. These agonists and antagonist didn’t have any significant effect on hot-plate latency time, when used alone. Neuroinflammation has a key contribution to brain disorders, especially learning-memory impairments. It has been shown that LPS-induced neuroinflammation can cause cognitive loss and hippocampus metabolic ailments29.
Studies suggest that LPS can induce neuroinflammatory responses and tauopathy-associated diseases30. It has been reported that Lyn agonist MLR-1023 pretreatment decreased cell apoptosis and inflammation31. Similarly, the data showed that LPS increased the TNF-α concentrations in the brain tissue as an inflammation marker. While MLR-1023 reduced the TNF-α concentrations. Hence, it can reduce the neuroinflammation that is made by LPS in the hippocampal tissue. Also, Pre-treatment with L-NAME increased the TNF-α concentrations. It means L-NAME has reduced the improving effect of MLR-1023 on neuroinflammation, made by LPS. While, L-arginine reduced the TNF-α concentrations, showing a potentiation in anti-inflammatory effect of MLR-1023 against LPS-induced neuroinflammation. Thus, it can be concluded that maybe NO has a role in the anti-inflammatory effect of MLR-1023 against LPS-induced neuroinflammation in the hippocampal tissue. Previously, it has been shown that levels of NO can increase amid neuroinflammation32.
Also based on our data, methylene blue increased the TNF-α concentrations. So, it has reduced the improving result of MLR-1023 on neuroinflammation made by LPS. However, sildenafil reduced the TNF-α concentrations, indicating an enhancement in the anti-inflammatory effect of MLR-1023 against LPS-induced neuroinflammation. Consequently, it can be inferred that maybe guanylate cyclase (GC)/cGMP path has a role play in the improving influence of MLR-1023 on neuroinflammation made by LPS. It confirms former reports; that cGMP accumulation has an anti-inflammatory roleplay32.
It has been newly reported that KATP channel opening can produce anti-inflammatory and analgesic effects33. Here, glibenclamide (a KATP channel blocker) caused TNF-α concentrations to rise, a decrease of the improving influence of MLR-1023 on neuroinflammation made by LPS. Whereas diazoxide (a KATP channel opener) didn’t have a significant effect. Subsequently, it can be supposed that maybe KATP channels are involved in the improving influence of MLR-1023 on neuroinflammation made by LPS. Accordingly, inhibiting of ATP-Sensitive Potassium Channels by LPS can lead to Neuroinflammation and Microglial Activation34.
Pioglitazone, a PPARγ receptors agonist, has shown improving effects on oxidative stress, neuroinflammation and cognitive loss in several ailments for example; Alzheimer’s disease. Moreover, regulation of TNF-α concentration as a pro-inflammatory cytokine has been suggested to be a potential mechanism35. Similarly, in this study pioglitazone reduced the TNF-α concentration, indicating an enhancement in the improving influence of MLR-1023 on neuroinflammation made by LPS. Although, GW9662 (a PPARγ receptors antagonist) increased the TNF-α concentrations, means; it can reduce the improving influence of MLR-1023 on neuroinflammation made by LPS. So, it can be thought that maybe PPARγ receptors are contributed to the improving influence of MLR-1023 on neuroinflammation made by LPS. These agonists and antagonists did not have a significant effect on TNF-α concentration when used alone.
CONCLUSION
The incidence of neurodegenerative disease has increased because of relationship between diabetes and Alzheimer’s disease. One of the issues that has attracted researchers’ attention is the mechanisms involved in brain insulin signaling and their relationship with Alzheimer’s disease. As previous studies have shown, MLR-1023 can recover insulin signaling and was recommended for diabetes dealing. This project’s results showed that MLR-1023 can improve depression, hyperalgesia and neuroinflammation induced by LPS in mice model and NO/cGMP/KATP channels signaling pathway and PPARγ receptors contributed to this influence.
SIGNIFICANCE STATEMENT
The prevalence of neurodegenerative disorders has increased due to the association between diabetes and Alzheimer’s disease. One of the issues that has attracted researchers’ attention is the mechanisms involved in brain insulin signaling and their relationship with Alzheimer’s disease. In this study, the effects of MLR-1023 on depression, hyperalgesia and hippocampal TNF-α level were studied in a model of Alzheimer’s disorder, produced by Lipopolysaccharide (LPS) injection. The role of PPARγ receptors and NO/cGMP/KATP-channels pathway was examined to determine likely mechanisms. These results showed that MLR-1023 can improve depression, hyperalgesia and neuroinflammation induced by LPS and KATP/cGMP/NO pathway and PPARγ receptors play a probable role. So, MLR-1023 and the mentioned mechanisms can be considered for the management of neurodegenerative diseases such as AD and new studies in this regard.
ACKNOWLEDGMENT
This paper is issued by financial support of the Vice Chancellor of Research, Dezful University of Medical Sciences (IR.DUMS.REC.1399.043), Dezful, Iran.
REFERENCES
- Zhang, W., J. Lu, Z. Qing, X. Zhang, H. Zhao, Y. Bi and B. Zhang, 2022. Effects of subcortical atrophy and Alzheimer’s pathology on cognition in elderly type 2 diabetes: The Alzheimer’s disease neuroimaging initiative study. Front. Aging Neurosci., 14.
- Fang, X.X., H. Wang, H.L. Song, J. Wang and Z.J. Zhang, 2022. Neuroinflammation involved in diabetes-related pain and itch. Front. Pharmacol., 13.
- Doroszkiewicz, J., P. Mroczko and A. Kulczyńska-Przybik, 2022. Inflammation in the CNS: Understanding various aspects of the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res., 19: 16-31.
- Shi, J., Q. Yin, L. Zhang, Y. Wu and P. Yi et al., 2022. Zi Shen Wan Fang attenuates neuroinflammation and cognitive function via remodeling the gut microbiota in diabetes-induced cognitive impairment mice. Front. Pharmacol., 13.
- Dolotov, O.V., L.S. Inozemtseva, N.F. Myasoedov and I.A. Grivennikov, 2022. Stress-induced depression and Alzheimer's disease: Focus on astrocytes. Int. J. Mol. Sci., 23.
- Botto, R., N. Callai, A. Cermelli, L. Causarano and I. Rainero, 2022. Anxiety and depression in Alzheimer's disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci., 43: 4107-4124.
- Aman, Y., T. Pitcher, C. Ballard and M. Malcangio, 2019. Impaired chronic pain-like behaviour and altered opioidergic system in the TASTPM mouse model of Alzheimer's disease. Eur. J. Pain, 23: 91-106.
- Ochman, A.R., C.A. Lipinski, J.A. Handler, A.G. Reaume and M.S. Saporito, 2012. The Lyn kinase activator MLR-1023 is a novel insulin receptor potentiator that elicits a rapid-onset and durable improvement in glucose homeostasis in animal models of type 2 diabetes. J. Pharmacol. Exp. Ther., 342: 23-32.
- Dolatshahi, M., S. Davoudi, Y. Paridar, R. Naserzadeh and B. Ghorbanzadeh, 2020. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of simvastatin in mice: Without tolerance and withdrawal syndrome. Neurosci. Lett., 714.
- Ghorbanzadeh, B., M.A. Behmanesh, R. Mahmoudinejad, M. Zamaniyan, S. Ekhtiar and Y. Paridar, 2022. The effect of montelukast, a leukotriene receptor antagonist, on the acetic acid-induced model of colitis in rats: Involvement of NO-cGMP-KATP channels pathway. Front. Pharmacol., 13.
- Dolatshahi, M., Y. Farbood, A. Sarkaki, S.M.T. Mansouri and A. Khodadadi, 2015. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson’s disease. Iran. J. Basic Med. Sci., 18: 38-46.
- Farbood, Y., A. Sarkaki, M. Dolatshahi, S.M.T. Mansouri and A. Khodadadi, 2015. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflammation in a rat model of parkinson’s disease. Basic Clin. Neurosci., 6: 83-90.
- Wang, Y., M. Wang, K. Fan, T. Li and T. Yan et al., 2018. Protective effects of alpinae oxyphyllae fructus extracts on lipopolysaccharide-induced animal model of Alzheimer's disease. J. Ethnopharmacol., 217: 98-106.
- Chen, F., A. Ghosh, F. Wu, S. Tang and M. Hu et al., 2017. Preventive effect of genetic knockdown and pharmacological blockade of CysLT1R on lipopolysaccharide (LPS)-induced memory deficit and neurotoxicity in vivo. Brain Behav. Immun., 60: 255-269.
- Shahsavarian, A., S. Javadi, S. Jahanabadi, M. Khoshnoodi and J. Shamsaeee,et al 2014. Antidepressant-like effect of atorvastatin in the forced swimming test in mice: The role of PPAR-gamma receptor and nitric oxide pathway. Eur. J. Pharmacol., 754: 52-58.
- Ludka, F.K., A.D.E. Zomkowski, M.P. Cunha, T. Dal-Cim, A.L.B. Zeni, A.L.S. Rodrigues and C.I. Tasca, 2013. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur. Neuropsychopharmacol., 23: 400-412.
- Ostadhadi, S., R. Akbarian, A. Norouzi-Javidan, V. Nikoui, S. Zolfaghari, M. Chamanara and A.R. Dehpour, 2017. Possible involvement of ATP-sensitive potassium channels in the antidepressant-like effects of gabapentin in mouse forced swimming test. Can. J. Physiol. Pharmacol., 95: 795-802.
- Remus, J.L. and R. Dantzer, 2016. Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol., 19.
- Sun, J., L. Qiu, H. Zhang, Z. Zhou, L. Ju and J. Yang, 2023. CRHR1 antagonist alleviates LPS-induced depression-like behaviour in mice. BMC Psychiatry, 23.
- Naserzadeh, R., N. Abad, B. Ghorbanzadeh, M. Dolatshahi and M.T. Mansouri, 2019. Simvastatin exerts antidepressant-like activity in mouse forced swimming test: Role of NO-cGMP-KATP channels pathway and PPAR-gamma receptors. Pharmacol. Biochem. Behavior., 180: 92-100.
- Kaster, M.P., P.K. Ferreira, A.R.S. Santos and A.L.S. Rodrigues, 2005. Effects of potassium channel inhibitors in the forced swimming test: Possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav. Brain Res., 165: 204-209.
- Yin, N., Q. Gao, W. Tao, J. Chen, J. Bi, F. Ding and Z. Wang, 2020. Paeoniflorin relieves LPS-induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. J. Leukocyte Biol., 108: 229-241.
- Li, H., S. Liu, Z. Wang, Y. Zhang and K. Wang, 2020. Hydrogen sulfide attenuates diabetic neuropathic pain through NO/cGMP/PKG pathway and μ-opioid receptor. Exp. Biol. Med., 245: 823-834.
- Li, D.Y., S.J. Gao, J. Sun, L.Q. Zhang and J.Y. Wu et al., 2023. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural Regener. Res., 18: 996-1003.
- Hajhashemi, V., H. Sadeghi and F.K. Madab, 2024. Anti-inflammatory and antinociceptive effects of sitagliptin in animal models and possible mechanisms involved in the antinociceptive activity. Korean J. Pain, 37: 26-33.
- Busserolles, J., C. Tsantoulas, A. Eschalier and J.A. López García, 2016. Potassium channels in neuropathic pain: Advances, challenges, and emerging ideas. PAIN, 157: S7-S14.
- Li, X., Q. Guo, Z. Ye, E. Wang and W. Zou et al., 2021. PPAR γ prevents neuropathic pain by down-regulating CX3CR1 and attenuating M1 activation of microglia in the spinal cord of rats using a sciatic chronic constriction injury model. Front. Neurosci., 15.
- Zhou, Y.Q., D.Q. Liu, S.P. Chen, N. Chen and J. Sun et al., 2020. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed. Pharmacother., 129.
- Schirmbeck, G.H., M. Seady, F.T. Fróes, J. Taday and C. da Ré et al., 2023. Long-term LPS systemic administration leads to memory impairment and disturbance in astrocytic homeostasis. NeuroToxicology, 99: 322-331.
- Park, J.H., J.W. Hwang, H.J.Lee, G.M. Jang and Y.J. Jeong et al., 2023. Lomerizine inhibits LPS-mediated neuroinflammation and tau hyperphosphorylation by modulating NLRP3, DYRK1A, and GSK3α/β. Front. Immunol., 14.
- Li, N., G. Lin, H. Zhang, J. Sun and M. Gui et al., 2023. Lyn attenuates sepsis-associated acute kidney injury by inhibition of phospho-STAT3 and apoptosis. Biochem. Pharmacol., 211.
- Rapôso, C., R.L. de Almeida Luna, A.K.S. Nunes, R. Thomé and C.A. Peixoto, 2014. Role of iNOS-NO-cGMP signaling in modulation of inflammatory and myelination processes. Brain Res. Bull., 104: 60-73.
- Qian, C., Y. Fan, L. Zong, C. Miao and L.L. Ji et al., 2023. Opening KATP channels induces inflammatory tolerance and prevents chronic pain. Brain Behav. Immun., 107: 76-86.
- Tang, Z., X. Shao, J. Wu, H. Chen and A. Zhang et al., 2021. Naloxone protects against lipopolysaccharide-induced neuroinflammation and microglial activation via inhibiting ATP-sensitive potassium channel. Comput. Math. Methods Med., 2021.
- Alhowail, A., R. Alsikhan, M. Alsaud, M. Aldubayan and S.I. Rabbani, 2022. Protective effects of pioglitazone on cognitive impairment and the underlying mechanisms: A review of literature. Drug Des. Dev. Ther., 16: 2919-2931.
How to Cite this paper?
APA-7 Style
Dolatshahi,
M., Hayay,
Y., Nazarinia,
D. (2024). NO/cGMP/KATP Pathway and PPAR Receptors Contribution in MLR-1023 Neuroprotection in LPS-Induced Mice Model of AD. Asian Journal of Biological Sciences, 17(4), 678-690. https://doi.org/10.3923/ajbs.2024.678.690
ACS Style
Dolatshahi,
M.; Hayay,
Y.; Nazarinia,
D. NO/cGMP/KATP Pathway and PPAR Receptors Contribution in MLR-1023 Neuroprotection in LPS-Induced Mice Model of AD. Asian J. Biol. Sci 2024, 17, 678-690. https://doi.org/10.3923/ajbs.2024.678.690
AMA Style
Dolatshahi
M, Hayay
Y, Nazarinia
D. NO/cGMP/KATP Pathway and PPAR Receptors Contribution in MLR-1023 Neuroprotection in LPS-Induced Mice Model of AD. Asian Journal of Biological Sciences. 2024; 17(4): 678-690. https://doi.org/10.3923/ajbs.2024.678.690
Chicago/Turabian Style
Dolatshahi, Mojtaba, Yasser Hayay, and Donya Nazarinia.
2024. "NO/cGMP/KATP Pathway and PPAR Receptors Contribution in MLR-1023 Neuroprotection in LPS-Induced Mice Model of AD" Asian Journal of Biological Sciences 17, no. 4: 678-690. https://doi.org/10.3923/ajbs.2024.678.690

This work is licensed under a Creative Commons Attribution 4.0 International License.


