Antibacterial Activities of Plant-Derived Metallic Nanoparticles on Some Selected Multidrug-Resistant Clinical Isolates
| Received 08 May, 2021 |
Accepted 15 Aug, 2021 |
Published 01 Jan, 2022 |
Background and Objective: Bacterial infections particularly with the emergence of multidrug-resistant (MDR) bacterial strains have become an urgent threat to public health. Noble metal nanoparticles (NPs), such as Silver Nanoparticles (Ag-NPs) and Copper-Oxide Nanoparticles (CuO-NPs) have been shown to exhibit strong and sustainable antibacterial action against a wide array of microorganisms. Hence, this research was aimed at evaluating the antibacterial effects of plant-derived bimetallic nanoparticles on some selected multidrug-resistant bacterial strains. Materials and Methods: The clinically isolated multidrug-resistant (MDR) bacteria, Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumonia were obtained from Sir Yahaya Memorial Hospital, Birnin Kebbi, Nigeria. The Ag-NPs and CuO-NPs were synthesized from the aqueous leaf extract of Carica papaya (ALECP). The green synthesis of the NPs was confirmed and characterized by the spectroscopic method, Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FT-IR) analysis. The synthesized NPs were also combined to formulate the bimetallic nanoparticles (BNPs). The antibacterial activity of the synthesized NPs and the formulated BNPs was evaluated using disc diffusion and broth dilution (MIC) methods. Results: The synthesized Ag- and CuO-NPs and the formulated BNPs have shown remarkable antibacterial activities against the tested clinically isolated multidrug-resistant (MDR) bacteria. Conclusion: Based on the findings of this research work, the Ag- and CuO-NPs as well as the formulated BNPs can be developed and used as alternatives for resolving or combating antibiotic resistance in the tested MDR bacterial strains.
| Copyright © 2022 Sani et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
An antibacterial agentis defined as a drug that at low concentration can selectively destroy bacteria or suppresses their growth or their ability to reproduce1. The over-use of antibiotics has led to the occurrence of antibiotic-resistant genes in various bacterial species. Antibacterial resistance is simply defined as “a microorganism's resistance to an antibacterial drug that was once able to treat an infection by that microorganism”2. Multiple drug resistance (MDR) is antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories3. The fast- spreading of drug-resistant infectious diseases poses a global health threat and notably, if the trend continues at the current speed, by 2050, it was predicted that 10 million people may die every year from drug-resistant infections4. To this extent, the design of novel and highly efficient antibacterial agents is needed. Different strategies, such as the use of nanotechnology are currently being developed to combat multidrug-resistant bacteria. The application of nanoparticles provides a potential strategy to overcome and manage the infections caused by the MDR bacteria5.
Nanoparticles (NPs) are particles of matter that are on a nano-scale, mostly between 1 and 100 nanometres (nm) in diameter. The nanoparticles may offer a promising solution to antibiotic resistance because, history teaches us that even before the advent of the antibiotic era, various types of inorganic substances were well known for their properties of killing or inhibiting bacterial growth6. The combination of the two different metal nanoparticles is what is referred to as bimetallic nanoparticles. The bimetallic nanoparticles may exhibit several new and improved properties. Due to their novel properties, they have gained a lot of attention among the scientific and industrial communities7.
Conventional approaches such as physical and chemical methods are used for the synthesis of nanoparticles8. However, these methods are associated with the use of heavy equipment, a huge amount of energy input and highly toxic and dangerous chemical compounds that generate biological hazards and most of the time these methods are not eco-friend and safe9.
Plant-mediated synthesis of nanoparticles seems to be very rapid, simple, dependable, non-toxic and eco-friendly10. The synthesis of metal nanoparticles using plant extracts deliver benefits over other biological synthesis methods which are associated with very difficult procedures such as maintaining microbial cultures11. The literature revealed that Carica papaya leaves extract contains phytochemicals that are efficiently capable of reducing metal ions to metal nanoparticles, thus, the biosynthesis of the Ag-NPs and CuO-NPs by using C. papaya leaf extract is possible and had been previously reported. Hence, this research aimed to evaluate the combined effect of plant-derived bimetallic nanoparticles on some selected multidrug-resistant bacterial strains.
MATERIALS AND METHODS
Study area: The research was conducted between September, 2021 and February, 2022 in Aliero Local Government, Kebbi State of Nigeria. It was performed in the Research Laboratories of the Departments of Biochemistry and Microbiology, Faculty of Life Sciences, Kebbi State University of Science and Technology, Aliero (KSUSTA), Nigeria.
Plant sample collection and authentication: The healthy leaves of Carica papaya were collected within Aliero Town, Kebbi State, Nigeria. The sample was authenticated in the Department of Plant Science and Biotechnology, Faculty of Life Sciences, KSUSTA, Nigeria.
Preparation of plant sample: Fresh leaves of C. papaya were washed thoroughly with distilled water and shade-dried. The dried leaves were powdered using mortar and pestle.
Preparation of sample for the synthesis of Ag-NPs: A 25 g of the powdered sample was percolated in 100 mL of distilled water in a conical flask, followed by boiling at 45°C for 30 min to facilitate the formation of aqueous extract. After reducing the temperature to ambient, the extract was filtered using Whatman No. 1 filter paper and the filtrate was stored at 4°C for further use12.
Preparation of sample for the synthesis of CuO-NPs: A 6 g of the powdered sample was percolated in 100 mL of distilled water in a conical flask, followed by boiling at 60°C for 10 min to facilitate the formation of aqueous extract. After reducing the temperature to ambient, the extract was filtered using Whatman filter paper No. 1 and the filtrate was stored at 4°C for further use13.
Qualitative phytochemical screening on the plant extract: This was carried out according to the methods described by Shaikh and Patil14 and Ayoola et al.15.
Synthesis of Ag-NPs: These were synthesized by the reduction method as described by Anbarasu et al.12. A 1 mM concentration of the silver nitrate solution was used. The solution was stored in an amber coloured bottle to prevent autoxidation of silver. About 10 mL of the prepared aqueous leaf extract of C. papaya and 90 mL of the 1 mM AgNO3 were firstly mixed. The mixture was then heated in a water bath at 60°C for 30 min till a colour change was observed. The formation of dark brown precipitate confirmed the formation of the nanoparticles12. The precipitate was centrifuged and washed several times with distilled water and dried at 50°C in a hot air oven for a day to obtain the pure nanoparticles.
Synthesis of CuO-NPs: These were synthesized by the reduction method as described by Turakhia et al.13. The 10 mM solution of copper sulphate pentahydrate (CuSO4.5H2O) was used for the synthesis of the CuO-NPs. A 5 mL of the prepared aqueous leaf extract of C. papaya and 95 mL of the 10 mM CuSO4.5H2O were mixed. The mixture was allowed to stand at ambient temperature till the colour changed. The formation of dark brown precipitate confirmed the formation of the nanoparticles13. The precipitate was centrifuged and washed several times with the distilled water and dried at 50°C in a hot air oven for a day to obtain the pure nanoparticles.
Characterization of the synthesized nanoparticles: Studies on the optical properties, morphology and the capping agent of the NPs were performed Utilizing Ultraviolet-Visible Spectroscopy (UV-Vis spectroscopy), Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FT-IR) analysis. The formation of the NPs and the involved mechanisms was studied from the data obtained.
UV-Vis analysis: The optical properties of the synthesised nanoparticles were studied using a Uv-vis spectrophotometer. The biosynthesis of Ag-NPs and the reduction of silver ions and Cu ions were analysed for Surface Plasmon Resonance (SPR) using ELICO SL-159 UV-visible spectrophotometer (Andhra Pradesh, India) for the spectrometric analysis of biosynthesized silver nanoparticles. The analysis was done in the wavelength range of 250-750 nm.
Scanning Electron Microscopy (SEM): The shape and morphology of the synthesized nanoparticles were studied by using the scanning electron microscope, JEOL Model (JSM-6390LV) instrument (USA).
Fourier Transform Infrared (FT-IR) analysis: The FT-IR analysis was carried out using Nicolet 6700, USA FT-IR spectrophotometer. The FT-IR spectra were collected from wavenumber (frequency) 3500-1500 cm–1.
Formulation of the bimetallic nanoparticles (BNPs): The BNPs (Ag-NPs+CuO-NPs) were formulated by physical mixing of the two prepared monometallic nanoparticles in the test tube as described by Zhang et al.16 with some modification.
Multidrug-resistant bacterial strains: The MDR Staphylococcus aureus, Klebsiella pneumonia and Pseudomonas aeruginosa were collected from Sir Yahaya Memorial Hospital Birnin Kebbi, Nigeria.
Antibiotic sensitivity test on the bacterial isolates: The sensitivity test was conducted using the disc diffusion method as described by Matuschek et al.17. Nutrient agar media was used to cultivate the bacterial strains. Fresh overnight inoculums (100 μL) of each culture were spread onto the nutrient agar plates. The standard antibiotic discs made by Maxicare Medical Laboratory were used. Each disc was made-up of ten small discs of 5 mm diameter containing ten different drugs. After overnight incubation at 37°C, zones of inhibition were assessed. The drug resistance is evaluated by the size of the clear zone and the greater the zone of inhibition, the lesser the bacterial drug resistance.
Antibacterial screening on the synthesized NPs and the formulated BNPs: The disc diffusion method as described by Reller et al.18 and Humphries et al.19 was used to screen the antibacterial effects on the multidrug-resistant isolates.
Determination of minimum inhibitory concentration (MIC): The tube dilution method was used for the determination of the MIC of the synthesized NPs as described by Owuama et al.20. Serial dilutions of the NPs were made in a liquid medium which was inoculated with a standardized number of organisms. The lowest concentration (highest dilution) of NPs preventing the appearance of turbidity was considered to be the minimal inhibitory concentration (MIC)21.
Determination of the synergistic effect: The checkerboard method was used to determine the synergistic effect of the BNPs as described by Odds21. The data obtained from the MIC assays were analysed in terms of the fractional inhibitory concentration (FIC). The fractional inhibitory concentration index (FICI) is the minimum inhibitory concentration (MIC) of the drug in combination divided by the MIC of the drug acting alone22.
RESULTS
The qualitative phytochemical screening of the aqueous leaf extract of the C. papaya (ALECP) revealed the presence of flavonoids, phenols, tannins, alkaloids and terpenoids as presented in Table 1. The UV absorption spectrometric analysis of the biosynthesized silver nanoparticles (Ag-NPs) and copper nanoparticles (CuO-NPs) was shown in Fig. 1 and 2, respectively. The maximum absorbance spectra at the wavelength between 300-500 nm confirmed the formation of the nanoparticles.
| Table 1: | Qualitative phytochemical composition of C. papaya leaf aqueous extract | |||
| Phytochemicals | Observation |
| Flavonoids | + |
| Phenols | + |
| Tannins | + |
| Saponins | - |
| Alkaloids | + |
| Terpenoids | + |
| +: Present and -: Not detected | |
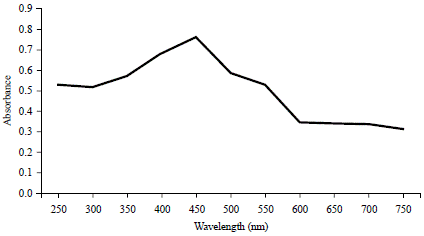 |
Fig. 1: Uv-Vis spectroscopy of the Ag-NPs |
 |
Fig. 2: Uv-Vis spectroscopy of the CuO-NPs |
 |
Fig. 3: Morphology of the synthesized Ag-NPs |
 |
Fig. 4: Morphology of the synthesized CuO-NPs |
The morphology of the biosynthesized Ag-NPs and CuO-NPs by the scanning electron microscopy was depicted in Fig. 3 and 4, respectively. The characterization of the biosynthesized Ag-NPs and CuO-NPs by FTIR revealed the functional groups of the capping agents of the NPs as seen in Fig. 5 and 6, respectively. The results of the antibiotic susceptibility test of the clinically isolated bacteria were presented in Table 2. From the result, K. pneumonia was recorded as the most resistant bacteria.
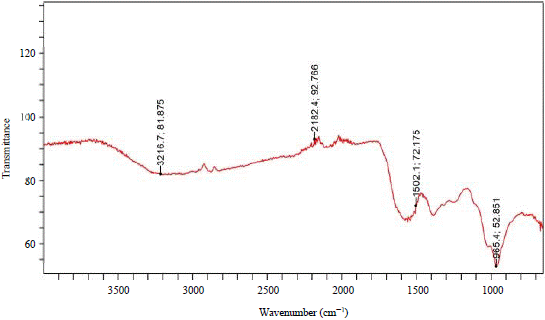 |
Fig. 5: FT-IR spectra of Ag-NPs |
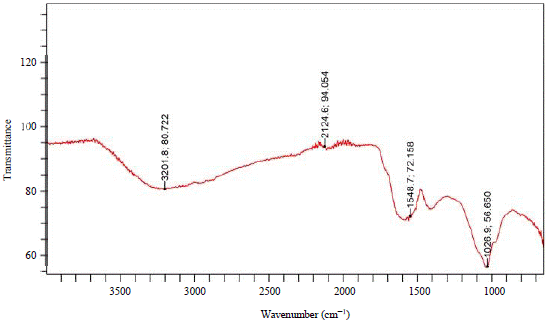 |
Fig. 6: FT-IR spectra of CuO-NPs |
| Table 2: | Antibiotic susceptibility profile of the test bacteria | |||
| Bacterium | SXT |
CH |
SP |
CPX |
AM |
AU |
CN |
PEF |
OFX |
S |
CRO |
| Gram-negative | |||||||||||
| K. pneumonia | R |
r |
R |
r |
r |
r |
r |
r |
r |
r |
i |
| P. aeruginosa | R |
r |
R |
s |
r |
r |
r |
s |
s |
r |
- |
| Gram-positive | |||||||||||
| PEF | CN |
APX |
Z |
AM |
R |
CPX |
S |
SXT |
E |
CRO |
|
| S. aureus | R |
r |
r |
R |
r |
r |
s |
r |
r |
r |
- |
| SXT: Septrin, CH: Chloramphenicol, SP: Sparfloxacin, CPX: Ciprofloxacin, AM: Amoxicillin, AU: Augmentin, CN: Gentamycin, PEF: Pefloxacin, OFX: Tarivid, S: Streptomycin, APX: Ampiclox, Z: Zinnacef, R: Rocephin, E: Erythromycin, CRO: Ceftriaxone, r: Resistant, s: Susceptible, i: Intermediate and -: Not tested | |||||||||||
The antibacterial activity of the synthesized NPs and formulated BNPs are presented in Table 3. From the result, the BNPs have shown remarkable antibacterial activity against the tested MDR bacteria. The result of the minimum inhibitory concentration (MIC) determination of the biosynthesized nanoparticles and the formulated bimetallic nanoparticles (BNPs) was presented in Table 4. From the result, the BNPs have shown the least MIC values compared to the monometallic NPs.
| Table 3: | Antibacterial activity of the metallic NPs | |||
Zone of inhibition (mm) |
||||||
| Bacterium | Ag-NPs (400 μg) |
CuO-NPs (400 μg) |
BNPS (800 μg) |
ALECP (400 μg) |
SD (30 μg) |
DW |
| K. pneumonia | 2.0±1.16 |
- |
7.0±1.76 |
- |
- |
- |
| P. aeruginosa | 12.0±1.15 |
7.0±1.76 |
23.0±0.58 |
- |
7.00±1.76 |
- |
| S. aureus | 12.0±0.88 |
11.0±0.67 |
25.0±0.67 |
- |
12.00±1.67 |
- |
Values are presented as Mean±SEM of triplicates, ALECP: Aqueous leaf extract of Carica papaya, SD: Standard drug (ceftriaxone was used for K. pneumonia, ciprofloxacin was used for P. aeruginosa and S. aureus, DW: Distilled water, -: No zone of inhibition was observed |
||||||
| Table 4: | MIC of the synthesized NPs and formulated BNPs on the test bacteria | |||
Minimum inhibitory concentration (μg mL–1) |
|||
| Bacterium | Ag-NPs |
CuO-NPs |
BNPs |
| K. pneumonia | 25 |
20 |
28.13 |
| P. aeruginosa | 25 |
25 |
3.13 |
| S. aureus | 12.5 |
12.5 |
1.57 |
| Table 5: | Fractional Inhibitory Concentration Index (FICI) of the BNPs | |||
| Bacterium | Fractional inhibitory concentration index |
| K. pneumonia | 0.25 |
| P. aeruginosa | 0.125 |
| S. aureus | 0.063 |
The Fractional Inhibitory Concentration Index (FICI) of the BNPs was calculated and presented in Table 5 to screen the synergistic effect of the BNPs.
DISCUSSION
The qualitative phytochemical screening of the Carica papaya leaf aqueous extract revealed the presence of tannins, phenols, flavonoids, alkaloids and terpenoids, which correspond to the result obtained by Ajiboye and Olawoyin22, who reported the presence of tannins, flavonoids, alkaloids, terpenoids and absence of saponins. The result is slightly different from the one reported by Anbarasu et al.12, who reported the positive results for tannins, saponins, phenols, flavonoids, alkaloids and terpenoids. They reported the presence of saponins which were reported as absent in this study. This might be due to the difference in geographical location. The phytochemicals detected in the extract act as reducing as well as capping or stabilizing agents in the green synthesis of the Ag-NPs and CuO-NPs as reported by Vaseghi et al.23.
In this study, the formation of Ag-NPs and CuO-NPs was confirmed by observing the colour change of the mixture and the optical properties of the nanoparticles using UV-vis spectrophotometry. The UV-vis spectrophotometric analysis is one of the most commonly used techniques for the characterization of synthesized NPs as well as for monitoring the stability and the formation of the nanoparticles. It involves quantifying the amount of ultraviolet or visible radiation absorbed by a constituent in a solution. Spectrophotometric absorption measurements in the wavelength ranges of 400-500 nm confirmed the formation of silver nanoparticles as reported by Huang and Yang24. Also Miri et al.25 reported the appearance of the Surface Plasmon Resonance (SPR) band between 400-500 nm confirmed the formation of the Ag-NPs. The maximum absorbance observed in this study at a wavelength of 450 nm for Ag-NPs is in line with the result obtained by Anbarasu et al.12, who reported the maximum absorbance of the Ag-NPs in UV-visible spectra at 445.7 nm. According to Khurana et al.26, the plasmonic characteristics are only observable when the particle size of metal nanoparticles is below its excitonic radius. The presence of the plasmon band in the UV-visible spectra of Ag-NPs indicates that the synthesized nanoparticles are small in size.
Noble metals are known to exhibit unique optical properties due to the property of Surface Plasmon Resonance (SPR)27. While, the surface plasmon resonance of CuO-NPs as observed from UV-visible spectrometry showed a sharp peak at 350 nm as seen in Fig. 2. Keabadile et al.28 reported that the UV absorption peak of CuO-NPs usually shows an absorption peak ranging from 280-360 nm. The result of this study is accorded with the result of John et al.29.
The Scanning Electron Microscopy (SEM) images justify the structural and morphological behaviour of the nanoparticles. From the SEM analysis, it was observed that most of the Ag-NPs have a spherical shape as presented in Fig. 3. The results for the CuO-NPs also showed that most of them are also spherical as presented in Fig. 4. The shape observed for the Ag-NPs is similar to the one obtained by Anbarasu et al.12, who synthesized Ag-NPs from the aqueous leaf extract of the Carica papaya. The shapes obtained for the CuO-NPs are also similar to the ones obtained by Bhavika et al.13, who synthesized CuO-NPs from the aqueous leaf extract of the Carica papaya.
The FT-IR analyses were carried out to identify the major functional groups on the surface of the nanoparticles and their possible involvement in the synthesis and stabilization of silver nanoparticles. The FT-IR result of Ag-NPs was presented in Fig. 5. The spectral bands appeared at 3216.7, 2182.4, 1502.1 and 965.4 cm–1. These bands were assigned to stretching vibration of O-H of phenols or flavonoids, S-CΞN of thiocyanate, N-O of nitro-compound and C=C of alkenes, while, the spectra of CuO-NPs were presented in Fig. 6. The bands appeared at 3201.8, 2124.6, 1548.7 and 1026.9 cm–1. These bands were also assigned to stretching vibration of O-H of phenols or flavonoids, S-CΞN of thiocyanate, N-O of nitro-compound and C=C of alkenes as interpreted by Ghaseminezhad et al.30. The similarity in the functional groups might be because, the Ag- and CuO-NPs were synthesized using the same plant extract. The phenols, flavonoids and thiocyanate that were detected, are among the major phytochemicals that were reported to interact with metal salts via their functional groups and mediate their reduction to nanoparticles and play roles in their stabilization Bar et al.31.
Table 3 presented the Ag-NPs showed remarkable antibacterial activity against K. pneumonia with a zone of inhibition of 2.00±1.16 mm, P. aeruginosa with a zone of inhibition of 12.0±1.15 mm and S. aureus with a zone of inhibition of 12.0±0.88 mm, while the CuO-NPs showed no activity (-) against the K. pneumonia, but effective against P. aeruginosa with a zone of inhibition of 7.00±1.76 mm and S. aureus with a zone of inhibition of 11.00±0.67 mm. From these results, it was observed that Klebsiella pneumonia was resistant to the 400 μg mL–1 of the CuO-NPs. This might be due to multiple resistant mechanisms possessed by the bacterial strain. The resistance might be explained by understanding the report of Parikh et al.32, who studied the mechanism of action of the CuO-NPs. They reported that the antibacterial effect of CuO-NPs was on the bacterial cell, thus, the nanoparticles have to get into bacterial cells in sufficient amounts before exerting their effect. In another word, Khurana et al.28 reported that the CuO-NPs diffuse into the bacterial cell either directly through pores or indirectly by ion channels or transport proteins. The Cu ions are released within the cell (through the Trojan horse mechanism) which in turn causes an increased concentration of reactive oxygen species (ROS) in the cells and consequently leads to cell death. This mechanism is similar to the mechanism of action of many drugs.
The Ag-NPs mostly exert their antibacterial effect on the cell surface, that is, through adhesion and accumulation on the bacterial cell surface, especially on Gram-negative bacteria. This may be due to the negative charge of the lipopolysaccharides (on the Gram-negative cell membrane) that promotes Ag-NPs adhesion as reported by Pal et al.33. The antibacterial activity observed for the Ag-NPs on both Gram-negative and Gram-positive bacteria is supported by the study conducted by Chauhan et al.34, Muñoz-Escobar et al.35 and Applerot et al.36. While, the antibacterial activity observed for the CuO-NPs on both Gram-negative and Gram-positive bacteria is in line with the study conducted by Vasantharaj et al.37 and Erci et al.38.
Table 3 and 4 presented the antibacterial activity of the bimetallic nanoparticles (BNPs). From the result, the 400 μg of the BNPs showed relatively higher activity against S. aureus (Gram-positive) with the zone of inhibition, 25.0±0.67 mm followed by P. aeruginosa (Gram-negative) with the zone of inhibition, 23.0±0.58 mm and then, the least activity was observed in K. pneumonia (Gram-negative) with the zone of inhibition, 7.00±1.76 mm as seen in Table 3. It was observed that the bimetallic nanoparticles (BNPs) showed remarkable antibacterial activities on the S. aureus, K. pneumonia and P. aeruginosa. This may be ascribed to the combined effects of the two individual nanoparticles. The BNPs displayed the highest inhibitory effect (25.0±0.67) against S. aureus. The difference in the effect of the BNPs among the Gram-positive and the Gram-negative may be due to the difference in the structure of their cell wall and the different effects of the individual nanoparticles as explained earlier.
The different mechanisms of antibacterial action of the two metal nanoparticles (Ag-NPs and CuO-NPs) contribute to the combined effect observed. As mentioned earlier, Ag-NPs exhibit antibacterial action mostly via a non-oxidative mechanism, which involves attacking the cell wall of the Gram-positive bacteria or cell membrane of the Gram-negative bacteria39, while, the CuO-NPs mostly cause the bacterial death through oxidative mechanism within the bacterial cells, which involves the generation of ROS, which consequently causes the oxidative damages on the cells40.
Table 5 shows the minimum inhibitory concentration (MIC) of Ag-NPs against K. pneumonia, P. aeruginosa and S. aureus was 25.00, 25.00 and 12.50 μg mL–1, respectively. While, the MIC of CuO-NPs against K. pneumonia, P. aeruginosa and S. aureus was 200.0, 25.00 and 12.50 μg mL–1, respectively. The MIC of Ag-NPs for K. pneumonia as recorded in this research is similar to the one reported by Banala et al.41, who reported the MIC of Ag-NPs as >25 μg mL–1 against the Gram-negative bacteria, K. pneumonia. The MIC of CuO-NPs for the K. pneumonia was recorded as the largest at 200 μg mL–1. This result is in line with the one obtained by Giannousi et al.42, who reported the MIC of CuO-NPs against Gram-negative bacteria, X. campestris and E. coli as >100 μg mL–1.
The MIC result of Ag-NPs against S. aureus was 12.50 μg mL–1. This is different from the result obtained by Ibrahim43, who reported the MIC of Ag-NPs as 5.1 μg mL–1 for the Gram-positive bacteria, S. aureus. The differences observed from the previous reports may be attributed to the different concentrations of the nanoparticles used as the initial concentration for serial dilution of the concentration in the broth dilution method (MIC determination method). The MIC of 12.50 μg mL–1 for CuO-NPs against S. aureus is in line with the one reported by John et al.31, who reported the MIC of CuO-NPs as 12.5-25 μg mL–1 against the Gram-positive bacteria, S. aureus.
Table 5 shows the MIC of BNPs against K. pneumonia, P. aeruginosa and S. aureus was recorded as 28.13, 3.13 and 1.57 μg mL–1, respectively. The MIC of BNPs for K. pneumonia was presented as the highest compared to the others. This may be due to the higher MIC of CuO-NPs for the K. pneumonia (200 μg mL–1) as stated earlier. Then, the Gram-positive bacteria, S. aureus was recorded to have the least MIC of 1.57 μg mL–1 compared to the others. This result has agreed with the one reported by Baker et al.44.
Table 6 shows the Fractional Inhibitory Concentration Index (FICI) of the BNPs against K. pneumonia, P. aeruginosa and S. aureus was 0.250, 0.125 and 0.063, respectively. The BNPs presented a strong synergistic effect against S. aureus with a FICI value of 0.063, followed by P. aeruginosa with a FICI value of 0.125 and then, the least synergistic effect was observed in K. pneumonia with FICI value of 0.250. All the FICI values were less than 0.5, thus, the combined effect was synergistic as interpreted by Fratini et al.45. If the FICI value is <0.5, the effect is said to be synergistic. If is >0.5-4, is said to be an additive effect, while, if it is >4, it has an antagonistic effect as reported by Magi et al.46.
CONCLUSION
Based on the findings of this research, the metallic nanoparticles have antibacterial activities against the tested MDR bacteria. The higher activity observed for the BNPs was found to be due to the synergic effect of the monometallic nanoparticles. This is attributed to the fact that each metal nanoparticle contributed through its unique mechanism of action.
SIGNIFICANCE STATEMENT
This study has discovered the potential of the synergistic effect of BNPs (Ag-NPS/CuO-NPs) to combat antibiotic resistance in the tested MDR bacteria. The pipeline of new antibiotics has been declining for several decades due to drug resistance, while many of the currently available antibiotics can no longer be effective against new MDR pathogens. Hence, this research would help researchers to develop a potent antibacterial agent from plant-derived BNPs that can combat and manage the spread of MDR bacteria.
REFERENCES
- Cerf, O., B. Carpentier and P. Sanders, 2010. Tests for determining in-use concentrations of antibiotics and disinfectants are based on entirely different concepts: “Resistance” has different meanings. Int. J. Food Microbiol., 136: 247-254.
- Li, B. and T.J. Webster, 2018. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res., 36: 22-32.
- Magiorakos, A.P., A. Srinivasan, R.B. Carey, Y. Carmeli and M.E. Falagas et al., 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect., 18: 268-281.
- Ruiz, J., 2021. Enhanced antibiotic resistance as a collateral COVID-19 pandemic effect? J. Hosp. Infect., 107: 114-115.
- Baptista, P.V., M.P. McCusker, A. Carvalho, D.A. Ferreira, N.M. Mohan, M. Martins and A.R. Fernandes, 2018. Nano-strategies to fight multidrug resistant bacteria-“A Battle of the Titans”. Front. Microbiol.
- Vert, M., Y. Doi, K.H. Hellwich, M. Hess and P. Hodge et al., 2012. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem., 84: 377-410.
- Sharma, G., A. Kumar, S. Sharma, M. Naushad, R.P. Dwivedi, Z.A. ALOthman and G.T. Mola, 2019. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci., 31: 257-269.
- Balasooriya, E.R., C.D. Jayasinghe, U.A. Jayawardena, R.W.D. Ruwanthika, R.M. de Silva and P.V. Udagama, 2017. Honey mediated green synthesis of nanoparticles: New era of safe nanotechnology.
- Natsuki, J., T. Natsuki and Y. Hashimoto, 2015. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl., 4: 325-332.
- Vijayaraghavan, K. and T. Ashokkumar, 2017. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng., 5: 4866-4883.
- Rajeshkumar, S. and L.V. Bharath, 2017. Mechanism of plant-mediated synthesis of silver nanoparticles-A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact., 273: 219-227.
- Anbarasu, A., P. Karnan, N. Deepa and R. Usha, 2018. Carica papaya mediated green synthesized silver nanoparticles. World J. Pharm. Res., 7: 397-407.
- Turakhia, B., M.B. Divakara, M.S. Santosh and S. Shah, 2020. Green synthesis of copper oxide nanoparticles: A promising approach in the development of antibacterial textiles. J. Coat. Technol. Res., 17: 531-540.
- Shaikh, J.R. and M.K. Patil, 2020. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud., 8: 603-608.
- Ayoola, G.A., H.A. Coker, S.A. Adesegun, A.A. Adepoju-Bello, K. Obaweya, E.C. Ezennia and T.O. Atangbayila, 2008. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res., 7: 1019-1024.
- Zhang, H., M. Haba, M. Okumura, T. Akita, S. Hashimoto and N. Toshima, 2013. Novel formation of Ag/Au bimetallic nanoparticles by physical mixture of monometallic nanoparticles in dispersions and their application to catalysts for aerobic glucose oxidation. Langmuir, 29: 10330-10339.
- Matuschek, E., D.F.J. Brown and G. Kahlmeter, 2014. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect., 20: 255-266.
- Reller, L.B., M. Weinstein, J.H. Jorgensen and M.J. Ferraro, 2009. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis., 49: 1749-1755.
- Humphries, R.M., S. Kircher, A. Ferrell, K.M. Krause, R. Malherbe, A. Hsiung and C.A.D. Burnham, 2018. The continued value of disk diffusion for assessing antimicrobial susceptibility in clinical laboratories: Report from the clinical and laboratory standards institute methods development and standardization working group. J. Clin. Microbiol.
- Owuama, C.I., 2017. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr. J. Microbiol. Res., 11: 977-980.
- Odds, F.C., 2003. Synergy, antagonism and what the chequerboard puts between them. J. Antimicrob. Chemother., 52: 1-1.
- Ajiboye, A.E. and R.A. Olawoyin, 2020. Antibacterial activities and phytochemical screening of crude extract of Carica papaya leaf against selected pathogens. Global J. Pure Appl. Sci., 26: 165-170.
- Vaseghi, Z., A. Nematollahzadeh and O. Tavakoli, 2018. Green methods for the synthesis of metal nanoparticles using biogenic reducing agents: A review. Rev. Chem. Eng., 34: 529-559.
- Huang, H. and X. Yang, 2004. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res., 339: 2627-2631.
- Miri, A., M. Sarani, M.R. Bazaz and M. Darroudi, 2015. Plant-mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties. Spectrochim Acta Part A: Mol. Biomol. Spectrosc., 141: 287-291.
- Khurana, C., P. Sharma, O.P. Pandey and B. Chudasama, 2016. Synergistic effect of metal nanoparticles on the antimicrobial activities of antibiotics against biorecycling microbes. J. Mater. Sci. Technol., 32: 524-532.
- Bindhu, M.R. and M. Umadevi, 2013. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc., 101: 184-190.
- Keabadile, O.P., A.O. Aremu, S.E. Elugoke and O.E. Fayemi, 2020. Green and traditional synthesis of copper oxide nanoparticles-comparative study. Nanomaterials.
- John, M.S., J.A. Nagoth, M. Zannotti, R. Giovannetti and A. Mancini et al., 2021. Biogenic synthesis of copper nanoparticles using bacterial strains isolated from an antarctic consortium associated to a psychrophilic marine ciliate: Characterization and potential application as antimicrobial agents. Mar. Drugs.
- Ghaseminezhad, S.M., S. Hamedi and S.A. Shojisadati, 2012. Green synthesis of silver nanoparticles by a novel method: Comparative study of their properties. Carbohydr. Polym., 89: 467-472.
- Bar, H., D.K. Bhui, G.P. Sahoo, P. Sarkar, S.P. De and A. Misra, 2009. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surfaces A: Physicochem. Eng. Aspects, 339: 134-139.
- Parikh, P., D. Zala and B.A. Makwana, 2014. Biosynthesis of copper nanoparticles and their antimicrobial activity. OALib.
- Pal, S., Y.K. Tak and J.M. Song, 2007. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol., 73: 1712-1720.
- Chauhan, N., A.K. Tyagi, P. Kumar and A. Malik, 2016. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol.
- Muñoz-Escobar, A., Á. de Jesús Ruíz-Baltazar and S.Y. Reyes-López, 2019. Novel route of synthesis of PCL-CuONPs composites with antimicrobial properties. Dose-Response.
- Applerot, G., J. Lellouche, A. Lipovsky, Y. Nitzan, R. Lubart, A. Gedanken and E. Banin, 2012. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small, 8: 3326-3337.
- Vasantharaj, S., S. Sathiyavimal, M. Saravanan, P. Senthilkumar and K. Gnanasekaran et al., 2019. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B: Biol., 191: 143-149.
- Erci, F., R. Cakir-Koc, M. Yontem and E. Torlak, 2020. Synthesis of biologically active copper oxide nanoparticles as promising novel antibacterial-antibiofilm agents. Prep. Biochem. Biotechnol., 50: 538-548.
- Lesniak, A., A. Salvati, M.J. Santos-Martinez, M.W. Radomski, K.A. Dawson and C. Åberg, 2013. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc., 135: 1438-1444.
- Li, Y., W. Zhang, J. Niu and Y. Chen, 2012. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano, 6: 5164-5173.
- Banala, R.R., V.B. Nagati and P.R. Karnati, 2015. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci., 22: 637-644.
- Giannousi, K., K. Lafazanis, J. Arvanitidis, A. Pantazaki and C. Dendrinou-Samara, 2014. Hydrothermal synthesis of copper based nanoparticles: Antimicrobial screening and interaction with DNA. J. Inorg. Biochem., 133: 24-32.
- Ibrahim, H.M.M., 2015. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci., 8: 265-275.
- Baker, S., P. Olga, R. Tatiana, P. Nadezhda and G. Tatyana et al., 2020. Phyto-nano-hybrids of Ag-CuO particles for antibacterial activity against drug-resistant pathogens. J. Genet. Eng. Biotechnol.
- Fratini, F., S. Mancini, B. Turchi, E. Friscia, L. Pistelli, G. Giusti and D. Cerri, 2017. A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. forst essential oils against Staphylococcus aureus strains. Microbiol. Res., 195: 11-17.
- Magi, G., E. Marini and B. Facinelli, 2015. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant group A Streptococci. Front. Microbiol.
How to Cite this paper?
APA-7 Style
Sani,
I., Ukwuani-Kwaja,
A.N., Abdulkadir,
D. (2022). Antibacterial Activities of Plant-Derived Metallic Nanoparticles on Some Selected Multidrug-Resistant Clinical Isolates. Asian Journal of Biological Sciences, 15(1), 15-26. https://doi.org/10.3923/ajbs.2022.15.26
ACS Style
Sani,
I.; Ukwuani-Kwaja,
A.N.; Abdulkadir,
D. Antibacterial Activities of Plant-Derived Metallic Nanoparticles on Some Selected Multidrug-Resistant Clinical Isolates. Asian J. Biol. Sci 2022, 15, 15-26. https://doi.org/10.3923/ajbs.2022.15.26
AMA Style
Sani
I, Ukwuani-Kwaja
AN, Abdulkadir
D. Antibacterial Activities of Plant-Derived Metallic Nanoparticles on Some Selected Multidrug-Resistant Clinical Isolates. Asian Journal of Biological Sciences. 2022; 15(1): 15-26. https://doi.org/10.3923/ajbs.2022.15.26
Chicago/Turabian Style
Sani, Ibrahim, Angela Nnenna Ukwuani-Kwaja, and Dahiru Abdulkadir.
2022. "Antibacterial Activities of Plant-Derived Metallic Nanoparticles on Some Selected Multidrug-Resistant Clinical Isolates" Asian Journal of Biological Sciences 15, no. 1: 15-26. https://doi.org/10.3923/ajbs.2022.15.26

This work is licensed under a Creative Commons Attribution 4.0 International License.


