Ameliorating Effect of Selected Vegetables on the Biochemical and Histological Changes in the Liver Associated with High-Calorie Diet
| Received 26 Jul, 2024 |
Accepted 11 Sep, 2024 |
Published 31 Dec, 2024 |
Background and Objective: The number of severe liver damage cases at the advanced stages of non-alcoholic fatty liver disease is increasing every year. The study determined the effect of Amaranthus hybridus, Crassocephalum crepidioides, Senecio biafrae and Corchorus olitorius on the biochemical and histological changes in the liver of male Wistar rats fed on a high-calorie diet. Materials and Methods: The vegetables’ leaves were picked, washed, cut and dried at 40°C. There were six groups of rats for the study. The positive control group was fed with food and water, while the negative control group received only a high-calorie diet. The four test groups were fed a high-calorie diet supplemented with 5% of one of the dried vegetables, respectively. At the end of five weeks, the liver weight, liver function test, oxidative stress and histology of the liver were determined. Data obtained were subjected to analysis of variance at p≤0.05 and means were separated using Duncan’s multiple range tests. Results: The liver weight was not significantly different except for the group fed on A. hybridus. There was a significant difference (p≤0.05) in the Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) levels among the groups. The group fed on S. biafrae (21.55 U/L) had significantly low AST, while ALT was significantly low in the group fed C. olitorius (25.48 U/L). Low levels of MDA (19.7 U/mL), SOD (16.4 U/mL) and CAT (16.53 U/mL) were recorded in the group fed S. biafrae. The histological derangement of the liver of the treatment groups was mild compared to the group fed a high-calorie diet. Conclusion: The vegetables had varying potentials for hepatoprotective. However, the S. biafrae group had better histology and the best hepatoprotective effect.
| Copyright © 2024 Kikelomo et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Non-Alcoholic Fatty Liver Disease (NAFLD) is a condition characterized by excessive fat accumulation in the liver, often linked to insulin resistance1. This can progress to serious complications like cirrhosis, liver cancer and heart disease. The increasing prevalence of NAFLD is associated with dietary factors2-4.
In the past decade, NAFLD has emerged as a major public health concern due to its association with advanced liver conditions like end-stage liver disease, liver failure and hepatocellular carcinoma5. With a global prevalence of approximately 24%, NAFLD affects various regions differently: 23.4% in Asia, 23.7% in Europe, 31.8% in the Middle East, 24.1% in North America, 30.5% in South America and 13.5% in Africa6. This high prevalence is likely linked to the rise in obesity, unhealthy high-calorie diets and sedentary lifestyles4. As obesity rates have surged, NAFLD has become the leading cause of chronic liver disease in Western countries4. Additionally, NAFLD is increasingly associated with an elevated risk of cardiovascular diseases, including coronary artery disease and peripheral vascular disease7. Consuming fiber-rich vegetables is an excellent nutritional supplement in high-calorie diets8. Many leafy vegetables are also valued for their medicinal properties and are used to treat various ailments9. Rich in antioxidants and phytochemicals, these vegetables can help prevent heart disease and stroke, control blood pressure and cholesterol levels, prevent certain types of cancer and combat other degenerative diseases10,11. Additionally, vegetables offer various health benefits that can positively influence liver health12. There is a need to thoroughly study their use to combat the menace of liver diseases. Thus, this study was carried out to determine the effect of A. hybridus, C. crepidioides, S. biafrae and C. olitorius on the liver biochemical and histological changes of male Wistar rats fed a high-calorie diet.
MATERIALS AND METHODS
Study area: The study was carried out at Babcock University, Nigeria and spanned through a period of eight months starting from September, 2019 to April, 2020. About 2 kg of the selected vegetable species (C. olitorius, C. crepidioides, A. hybridus and S. biafrae) were obtained from major markets in Western Nigeria. The vegetables were picked, washed, shredded and dried in the oven at 40°C4. The dried vegetables were blended, packed in polyethylene bags and stored at 4±2°C until they were required for use.
High-calorie diet: Commercially available vegetable oil (refined palm olein) was added to standard rat feed at a concentration of 30% to increase the calorie content13 and the drinking water also contained a 30% concentration of sucrose14. The composition of the oil was such that transfat was <1 g, saturated fat 50 g, Monounsaturated (min) 38 g, omega 9 fatty acid 38 g, vitamin A 800 μg (100% of the RDI) and vitamin E 20 mg (167% of the RDI).
Animal studies: A total of 30 male Wistar rats obtained from Babcock University Animal Facility that were 4 weeks old were housed in cages for two weeks to acclimatize under the prevailing atmospheric conditions (27±2°C) with access to standard rat feed and water only. At the beginning of the experiment, the rats were randomly divided into 6 groups (n = 5), which consisted of 2 control and 4 test groups. Each rat in these different groups was marked and the groups were coded for identification. Group A (positive control) was given standard rat feed with water while group B (negative control) was given high-calorie diet (feed with vegetable oil and water containing sucrose). The test groups were all given high-calorie diet alongside the vegetables. Group C was given C. olitorius while group D was given C. crepidioides. Also, group E was given A. hybridus while group F was fed S. biafrae. The vegetables were added to the feed at a concentration of 5%, respectively15.
The rats were deprived of food for 24 hrs after the last treatment. The ocular puncture method was used to collect blood samples from the rats in the different groups into heparin bottles for liver function tests. The rats were rendered unconscious through cervical dislocation and dissected to remove the liver.
The collected three liver samples from each of the groups were weighed and preserved afterward in 10% saline phosphate buffer solution and 10% formalin solution (tissue fixation) for oxidative stress and histopathological analysis, respectively. The test rats were handled following the guidelines of the National Institutes of Health (NIH) and ethical approval (NHREC/24/01/2018) for the research protocol was sought from Babcock University Health Research Ethics (BUHREC).
Determination of alanine aminotransferase and aspartate aminotransferase: The ALT and AST kits by Randox Laboratories Limited (County Antrim, BT29 4QY, United Kingdom) were used and the protocol as described in the manual was followed. The ALT and AST reagents (0.5 mL) containing 100 mmol/L of phosphate buffer at pH of 7.4, 2.0 mmol/L of α-oxoglutarate and 200 mmol/L of either L-alanine or L-aspartate were pipetted into clean, dried test tubes properly labeled as blanks and samples separately. Distilled water (0.1 mL) was pipetted into the test tubes labeled as blanks and 0.1 mL of the various blood samples were placed in their appropriate samples’ test tubes. The content of the various test tubes was properly mixed and incubated for exactly 30 min at 37°C.
After incubation, 0.5 mL of 2.0 mmol/L 2,4-dinitrophenylhydrazine was added to all test tubes. The content of the various test tubes was mixed and allowed to stand for exactly 20 min at 25°C. About 5.0 mL of sodium hydroxide was dispensed into all test tubes and the content of the test tube was mixed. The absorbance (546 nm) of the samples was read against the reagent blank after 5 min using a spectrophotometer (752S UV-visible). The activities of serum ALT and AST were obtained from the table provided in the Randox laboratory manual for the determination of ALT and AST in the serum15.
Oxidative stress biomarkers: The rats' livers were quickly removed and immediately the blood was washed out with ice-cold 0.9% saline solution before weighing and storing at -20°C. Homogenates of the tissues were prepared with 1.0 g/10 mL in 250 mM sucrose, 1 mM EDTA, 1 mM DL-dithiothreitol and 15 mM Tris-HCl at pH 7.4 using a homogenizer (GLH 850). Each homogenate was centrifuged at 800 g for 30 min at 4°C. The resulting supernatant fraction was used to determine enzyme activity and MDA levels. The protein concentrations of the supernatant were determined by the method described by Bradford16.
Malondialdehyde (MDA) levels: Estimates of lipid peroxidation levels were evaluated by the Thiobarbituric Acid Reactive Substances (TBARS) procedure, described by Tsikas17 on homogenized tissues. Two millimeters of distilled water was added to 0.1 mL of the tissue sample, followed by 1 mL of TBA reagent and 1 mL of trichloroacetic acid. The mixture was then heated in a boiling water bath for 10 min before adding butanol. After cooling, the mixture was centrifuged for 10 min. The absorbance of the organic phase was measured at 532 nm and compared to the blank.
Superoxide dismutase (SOD) activity: The SOD activity was determined using quercetin as the substrate following the dilution method described by Raguvaran et al.18. The assay mixture, totaling 1 mL, consisted of 0.1 mol/L sodium phosphate buffer (pH 7.8) and 0.08 mmol/L EDTA in a 1:1 ratio. After dilution, 0.1 mL of the tissue sample (1:1000) was added to 2.3 mL of distilled water, followed by 1 mL of the assay mixture containing EDTA and sodium phosphate buffer. The increase in absorbance due to quercetin oxidation was measured spectrophotometrically at 406 nm at 0 and 20 min. In the blank sample, the tissue sample was replaced with an equal amount of distilled water. One unit of SOD activity was taken as the amount of enzyme that stopped the oxidation of quercetin by 50% under the prevailing experimental conditions.
Catalase (CAT) activity: The CAT activity was measured using the method described by Melekh et al.19. The reaction was initiated by adding 0.1 mL of the tissue sample to 1 mL of 4% ammonium molybdate and 2 mL of 0.03% hydrogen peroxide (H2O2) solution. One unit of catalase activity is defined as the amount of enzyme required to decompose 1 μmol of H2O2 per minute per gram of tissue. The breakdown of hydrogen peroxide was monitored to determine the enzyme activity in the reaction mixture was measured spectrophotometrically at 410 nm.
Liver histopathology: The tissue (liver) samples were removed from the fixative bottles and small pieces were trimmed out and mounted on the slides. The slides were placed in xylene solution (changed three times) for 3 min each. Tissue sections were then hydrated by immersing them in decreasing concentrations of alcohol (100 and 95%), with each concentration change lasting 3 min. They were subsequently rinsed in distilled water until ripples disappeared from the slides. The slides were then stained with hematoxylin for 8-15 min, followed by washing under running water until clear.
Next, the slides were dipped in a 1% hydrochloric acid alcohol differentiation solution with 3-6 quick dips for about 5-30 sec until the slides turned red. They were then rinsed in water for about 15 min until the sections appeared blue. The differentiation was checked microscopically to ensure distinct nuclei and uncolored cytoplasm. The tissue sections were then immersed in an alkaline solution (Bluing Agent) for about 3-5 min and washed in lukewarm water 5 min later.
The tissues were stained with eosin for 30 sec to 2 min. The sections were then dehydrated in increasing concentrations of alcohol (95 and 100%), with 3 changes lasting 2 min each. Finally, the tissues were cleared in 3 changes of xylene for 2 min each and a cover glass was mounted20.
Statistical analysis: The analyses were carried out in triplicate and the results were recorded as Mean±SD. The difference among the groups was determined by subjecting the data to a one-way analysis of variance (p≤0.05) and separating the means by Duncan's multiple range test.
RESULTS
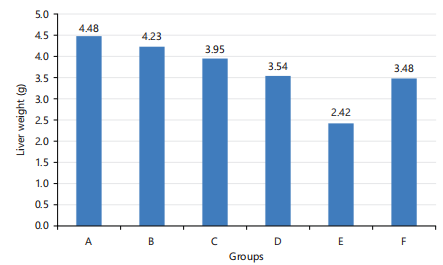
Liver weight: Liver weight results were presented in Fig. 1. The liver weight of all groups was not significantly different except for the group fed A. hybridus. The weight of the liver ranged between 2.42±0.38 and 4.48±0.70 g with the group fed A. hybridus having a liver weight of 2.42 g which was significantly (p≤0.05) smaller than other liver samples. However, the liver weight of the entire test group and the negative control group was insignificantly smaller than that of the positive control group (4.48 g).
|
|
|
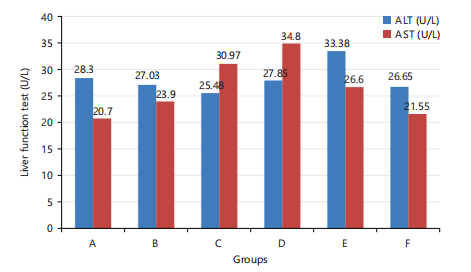
Liver function test: Figure 2 presents liver function test results where the ALT mean ranged from 25.48±3.27 to 33.38±4.40 U/L and the group fed C. olitorius had the least value which was significantly lower (p≤0.05) than what was recorded for the positive control group (28.30±4.80 U/L). For the AST, the mean range was from 21.55±2.26 to 34.80±0.27 U/L for the test groups with the group fed S. biafrae having a value comparable to 20.70±3.18 U/L for the positive control group at p≤0.05. It was observed that there was a significant difference (p≤0.05) in AST and ALT among all groups.
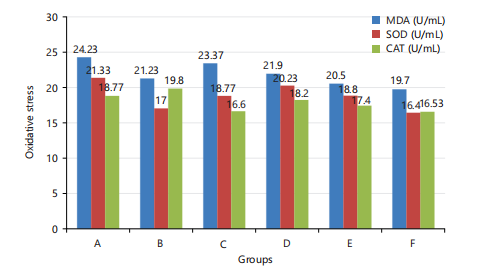
Oxidative stress indices: The results of oxidative stress markers of the liver samples are represented in Fig. 3. The MDA mean range was between 19.70±4.25 and 24.23±5.50 U/mL, the CAT mean ranged between 16.53±3.58 and 19.80±5.50 U/mL and the SOD mean range was between 16.4±2.89 and 21.33±4.23 U/mL for the control and the test groups.
|
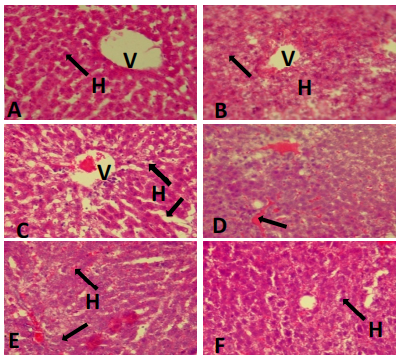
Liver histopathology: Figure 4 shows the photomicrograph of the liver and the arrows point to features in the hepatocytes. Group A (positive control) reveals a near-perfect histoarchitecture with a non-congested central vein. Groups B, D and E on the other hand show histological derangement as features of necrosis can be seen in the hepatocytes with mild hemorrhagic features in groups D and E (arrow). Group C shows distinct hepatocytes but dilated sinusoids (arrow). Group F (S. biafrae) shows better histology when compared with other treated groups.
DISCUSSION
There was a reduction in the liver size of all the test groups compared to the negative control though the positive control had higher liver size which may be due to higher consumption of feed. High-calorie feed increased liver size in the negative control which was reduced by the vegetables with a greater reduction in the group fed on A. hybridus. The A. hybridus has the potential to reduce serum triglycerides which may consequently reduce the liver weight21.
Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) are involved in metabolism and can help predict the functionality of the liver. For AST, the positive control group and the group fed S. biafrae had significantly lower values of 20.70 and 21.55 U/L, respectively, which corroborated the reports of Okoro and Kadiri22 of the positive effect of S. biafrae on the liver of test rats. However, for ALT, the difference between the positive control group and the test groups was not pronounced as only the group fed A. hybridus had significantly high ALT (33.38 U/L) while ALT of the group fed C. olitorius was significantly low (25.48 U/L). With the observation made on the level of AST in particular, there is a possibility of an onset of metabolic abnormalities in the liver of the rats in the test groups and the negative control group as elevated levels of this enzyme and ALT indicate metabolic abnormalities23,24. In addition, the rats in the negative and the test groups were found to be less active when compared to the rats in the positive control group, which may be a sign of lethargy as reported by Swain25.
Liver diseases are associated with an increase or decrease in antioxidant levels. Oxidative stress happens as a result of the production of reactive oxygen species faster than they can be safely neutralized by antioxidant mechanisms and/or by a decrease in antioxidant defense26. Enzyme activities in tissues are usually used as markers to ascertain the toxic effects of administered foreign compounds on experimental animals24. The result of lipid peroxidation showed an increased concentration of MDA in the positive control group compared to other groups. While the levels of CAT and SOD were higher in the negative control group and the positive control group, respectively, though not significantly at p≤0.05. Group-fed S. biafrae recorded the lowest levels of MDA, CAT and SOD. The reduction of the oxidative stress index portrays the ability of this vegetable to oppose the precipitation of liver disease. This finding supports the report of Okoro and Kadiri22 on the hepatoprotective effects of S. biafrae.
The nonsignificant difference in the oxidative stress indices may be attributed to the low saturated fat, presence of polyunsaturated fat, omega 9 and high vitamin E in the oil used for the high-calorie diet.
According to Oh et al.27, membranes that are rich in Monounsaturated Fatty Acids (MUFA) are less susceptible to oxidation and omega 6, omega 9 and vitamin E confer protection against lipid peroxidation of different polyunsaturated fatty acids. These antioxidants decrease the accumulation of reactive oxygen species, which reduces local tissue damage and accelerates the healing process28. The composition of the cooking oil used seemed to reduce the risk of oxidative stress otherwise associated with the consumption of a high-calorie diet. This may also be responsible for the results obtained in the liver function test.
Histopathology of the liver samples of the test animals revealed a normal structure of the liver of the positive control group while the negative control group showed histological derangement as features of necrosis which is mild in groups fed on C. crepidioides and A. hybridus. This finding was supported by the report of Adeoye et al.4 on the effect of vegetables on the liver. However, it is contrary to the report of Aniya et al.29 on the effect of C. crepidioides on the liver and the report of Balasubramanian and Karthikeyan30 of A. hybridus possessing significant nephron protective effect against oxidative damage in diabetic rats.
Another finding is that the group fed C. olitorius portrayed distinct hepatocytes but also had dilated sinusoids. This observation supported the report of Omeje et al.31 on hepatoprotective properties of ethanol extract of C. olitorius. However, Iweala and Okedoyin32 observed that regular consumption of unprocessed C. olitorius may further enhance the hepatotoxic potential of carbon tetrachloride (CCl4).
The group fed S. biafrae had generally better histology when compared with other treatment groups. This was in support of the report of Muhammed et al.33 where the histological observations of the sections of the frontal cortex, liver, kidney and testis of experimental rats administered aqueous leaf extract of S. biafrae revealed no deleterious effect on all organs and there were no histopathological alterations.
CONCLUSION
Senecio biafrae significantly lowered the level of AST enzyme and Corchorus olitorius also had a lowering effect on the ALT enzyme. Senecio biafrae had a lowering effect on the oxidative stress indices (MDA, CAT and SOD). Amaranthus hybridus group had low liver weight compared to the rest of the test groups. The liver of the group fed on S. biafrae showed good histology with no histopathological alterations compared to the negative control group and the other three test groups. Senecio biafrae was more effective in ameliorating the effect of a high-calorie diet on the liver, while other vegetables also exhibited potential.
SIGNIFICANCE STATEMENT
The effects of Amaranthus hybridus, Crassocephalum crepidioides, Senecio biafrae and Corchorus olitorius on the liver of Wistar rats fed on a high-calorie diet were compared to that of rats on a standard diet and rats on a high-calorie diet alone without vegetables. It was found that S. biafrae had the strongest protective effect on the rats' livers. Rats that ate S. biafrae had lower levels of AST and ALT enzymes and oxidative stress. Corchorus olitorius displayed the ability to lower ALT and A. hybridus prevented excessive liver weight. The histology of the liver tissues of the vegetable groups revealed healthier structures. The study suggests these vegetables hold promise as dietary interventions to mitigate liver disease risks associated with high-calorie diets.
ACKNOWLEDGMENT
The authors are immensely grateful to Babcock University, Nigeria, for granting permission to use the animal facility and the laboratory. They are also grateful to Mr. Taiye Adelodun for the interpretation of the liver photomicrograph.
REFERENCES
- Ali, R. and K. Cusi, 2009. New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann. Med., 41: 265-278.
- Rinella, M.E. and A.J. Sanyal, 2016. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol., 13: 196-205.
- de Alwis, N.M.W. and C.P. Day, 2008. Non-alcoholic fatty liver disease: The mist gradually clears. J. Hepatol., 48: S104-S112.
- Adeoye, B.K., S.O. Adeyele, J.A. Adeyeye, O.O. Oyerinde, M.F. Olanrewaju and I.F. Ani, 2019. Therapeutic effect of white cabbage (Brassica oleracea) aqueous extract on hyperglycemia in prediabetes-induced male albino rats. J. Appl. Sci., 19: 413-420.
- Adams, L.A., J.F. Lymp, J.S. Sauver, S.O. Sanderson, K.D. Lindor, A. Feldstein and P. Angulo, 2005. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology, 129: 113-121.
- Younossi, Z.M., A.B. Koenig, D. Abdelatif, Y. Fazel, L. Henry and M. Wymer, 2016. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology, 64: 73-84.
- Targher, G., C.P. Day and E. Bonora, 2010. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med., 363: 1341-1350.
- Adeoye, B.K., A.O. Oduko, A.O. Adeoye, K. Ayodele and N.C. Uwannah et al., 2022. Feeding behaviour, weight gain and blood sugar of male Wistar rats fed on a high-calorie diet and vegetables. Afr. J. Food Agric. Nutr. Dev., 22: 21127-21145.
- Dada, T.E., K. Otitoloju, R. Adjonu, J. Crockett and E.U. Nwose, 2021. Nutritional and medicinal values of common green leafy vegetables consumed in Delta State, Nigeria: A review. Int. J. Community Med. Public Health, 8: 2564-2571.
- Fasuyi, A.O., 2006. Nutritional potentials of some tropical vegetable leaf meals: Chemical characterization and functional properties. Afr. J. Biotechnol., 5: 49-53.
- Adeoye, B.K., S.O. Adeyele, E.O. Ngozi, A.R. Akinlade and I.F. Ani, 2019. Effect of white cabbage (Brassica oleracea) aqueous extract on oxidative stress in pre-diabetes-induced male albino rats. Niger. J. Nutr. Sci., 40: 63-72.
- Uprety, Y., H. Asselin, E.K. Boon, S. Yadav and K.K. Shrestha, 2010. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed., 6.
- Nampurath, G.K., S.P. Mathew, V. Khanna, R.T. Zachariah, S. Kanji and M.R. Chamallamudi, 2008. Assessment of hypolipidaemic activity of three thiazolidin-4-ones in mice given high-fat diet and fructose. Chem. Biol. Interact., 171: 363-368.
- Judith, H., C. Lynette and R. Sheldon, 1981. Effects of feeding rats sucrose in a high fat diet. J. Nutr., 111: 531-536.
- Adeoye, B.K., Z.O. Alonge, M.D. Olumide, I.F. Ani, M.F. Olanrewaju, E.O. Ngozi and O.O. Oyerinde, 2019. Effect of cinnamon (Cinnamomum cassia) on blood sugar, lipid profile and liver function of male Wistar rats. Pak. J. Nutr., 18: 989-996.
- Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248-254.
- Tsikas, D., 2017. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem., 524: 13-30.
- Raguvaran, K., M. Kalpana, T. Manimegalai, S. Kalaivani and P. Devapriya et al., 2022. Larvicidal, antioxidant and biotoxicity assessment of (2-(((2-ethyl-2 methylhexyl)oxy)carbonyl)benzoic acid isolated from Bacillus pumilus against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. Arch. Microbiol., 204..
- Melekh, B., I. Ilkiv, A. Lozynskyi and A. Sklyarov, 2017. Antioxidant enzyme activity and lipid peroxidation in rat liver exposed to celecoxib and lansoprazole under epinephrine-induced stress. J. Appl. Pharm. Sci., 7: 94-99.
- Sheehan, D.C. and B.B. Hrapchack, 1987. Theory and Practice of Histotechnology. 2nd Edn., Battelle Press, Columbus, Ohio, ISBN: 9780801645730, Pages: 481.
- Adeoye, B.K., G.O. Dada, O.O. Oyerinde, A.R. Akinlade, I. Esiaba and O.A. Adewole, 2022. Weight change, haematology and lipid profile of normal male Wistar rats fed on high-calorie diet and vegetables. Niger. J. Nutr. Sci., 43: 161-171.
- Okoro, I.O. and H.E. Kadiri, 2019. Anti-oxidant and hepatoprotective effects of Senecio biafrae on CCl4-induced liver damage in rats. Iran. J. Toxicol., 13: 31-35.
- Perera, S., V. Lohsoonthorn, W. Jiamjarasrangsi, S. Lertmaharit and M.A. Williams, 2008. Association between elevated liver enzymes and metabolic syndrome among Thai adults. Diabetes Metab. Syndr.: Clin. Res. Rev., 2: 171-178.
- Adesokan, A.A., O.I. Oyewole and B.M.S. Turay, 2009. Kidney and liver function parameters in alloxan-induced diabetic rats treated with aloe barbadensis juice extract. Sierra Leone J. Biomed. Res., 1: 33-37.
- Swain, M.G., 2006. Fatigue in liver disease: Pathophysiology and clinical management. Can. J. Gastroenterol., 20: 181-188.
- Favier, A., 2006. Oxidative stress in human diseases. Ann. Pharm. Fr., 64: 390-396.
- Oh, Y.T., J.Y. Lee, J. Lee, H. Kim, K.S. Yoon, W. Choe and I. Kang, 2009. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: Possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-κB signaling pathways. Neurosci. Lett., 464: 93-97.
- Niemoller, T.D. and N.G. Bazan, 2010. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediators, 91: 85-89.
- Aniya, Y., T. Koyama, C. Miyagi, M. Miyahira, C. Inomata, S. Kinoshita and T. Ichiba, 2005. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol. Pharm. Bull., 28: 19-23.
- Balasubramanian, T. and M. Karthikeyan, 2016. Therapeutic effect of Amaranthus hybridus on diabetic nephropathy. J. Dev. Drugs, 5.
- Omeje, K., O. Henry, O. Arome, A. Ogechukwu and U. Chimere, 2016. Liver enzymes and lipid activities in response to Corchorus olitorius leaf extract. Int. J. Curr. Res. Biosci. Plant Biol., 3: 45-49.
- Iweala, E.E.J. and A.G. Okedoyin, 2014. Effect of consumption of Corchorus olitorius L., in carbon tetrachloride induced liver damage in male Wistar rats. Am. J. Biochem. Mol. Biol., 4: 143-154.
- Muhammed, A.O., D.A. Adekomi and A.A. Tijani, 2012. Effects of aqueous crude leaf extract of Senecio biafrae on the histology of the frontal cortex, kidney, liver and testis of male Sprague Dawley rats. Sci. J. Biol. Sci., 1: 13-18.
How to Cite this paper?
APA-7 Style
Kikelomo,
A.B., Oluwaferanmi,
S.K., Adetayo,
A.O., Adegoke,
A.S., Tirzah,
A. (2024). Ameliorating Effect of Selected Vegetables on the Biochemical and Histological Changes in the Liver Associated with High-Calorie Diet. Asian Journal of Biological Sciences, 17(4), 754-762. https://doi.org/10.3923/ajbs.2024.754.762
ACS Style
Kikelomo,
A.B.; Oluwaferanmi,
S.K.; Adetayo,
A.O.; Adegoke,
A.S.; Tirzah,
A. Ameliorating Effect of Selected Vegetables on the Biochemical and Histological Changes in the Liver Associated with High-Calorie Diet. Asian J. Biol. Sci 2024, 17, 754-762. https://doi.org/10.3923/ajbs.2024.754.762
AMA Style
Kikelomo
AB, Oluwaferanmi
SK, Adetayo
AO, Adegoke
AS, Tirzah
A. Ameliorating Effect of Selected Vegetables on the Biochemical and Histological Changes in the Liver Associated with High-Calorie Diet. Asian Journal of Biological Sciences. 2024; 17(4): 754-762. https://doi.org/10.3923/ajbs.2024.754.762
Chicago/Turabian Style
Kikelomo, Adeoye,, Bolade, Sadiku Kafayat Oluwaferanmi, Adewole, Oluwaseun Adetayo, Adewole Samuel Adegoke, and Alfa, Tirzah.
2024. "Ameliorating Effect of Selected Vegetables on the Biochemical and Histological Changes in the Liver Associated with High-Calorie Diet" Asian Journal of Biological Sciences 17, no. 4: 754-762. https://doi.org/10.3923/ajbs.2024.754.762

This work is licensed under a Creative Commons Attribution 4.0 International License.



