Acetaminophen-Induced Infertility in Male Wistar Rats: Impact of Avocado Leaves on Hormonal Profile and Semen Analysis
| Received 19 Sep, 2024 |
Accepted 29 Oct, 2024 |
Published 31 Mar, 2025 |
Background and Objective: Male infertility is intended as a condition for men who are unable to impregnate their partners for at least one year after intercourse without using a protector. This research work statistically investigates the effects of paracetamol usage on hormonal profile and sperm count by modelling the impact of paracetamol and standard drug, same dosage of paracetamol combinations and the interactions on hormonal profile and sperm count and to determine the effect of consuming avocado on the parameters. Materials and Methods: Forty-two male Wistar rats weighing 100-105 g were used to determine the effect of acetaminophen on hormones and sperm count. The avocado leaves were air-dried and blended into powder, mixed with water (100 mL), stirred and mixed intermittently for 72 hrs. The water extract was filtered into a conical flask and stored until when used. The animals were humanely sacrificed after which a caudoventral mid-abdominal incision was made to access the internal organs. The testes were detached from the epididymis and harvested. The concentration was determined by the use of the improved Neubauer hemocytometer. Results: The LH and FSH hormones in the groups apart from the control increased significantly due to acetaminophen administration but the administration of standard dose, helped in reviving the damage to a significant level. However, long-term administration of acetaminophen doesn’t reduce body weight, avocado extract reduces it to a significant level. However, an excessive increase in FSH concentration as observed in the 3000 mg/kg of acetaminophen treated rats may negatively affect reproduction due to its effect on spermatogenesis. As observed, testosterone levels in the lower doses increased but decreased in the high-dose group. Conclusion: The result showed that avocado had a negatively significant influence on semen, sperm motility and viability were reduced and the consumption of high amount of avocado leaf extract has a negative effect on the reproductive system in the male.
| Copyright © 2025 Stanley et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Male infertility is defined as the inability of a man to have a child after sexual activity for a minimum of one year without the use of a protector1. Although both the male and female partners can contribute to infertility, an estimated 50% of cases are caused entirely or largely by male factors2-6. An individual becomes infertile when sperm are unable to fertilize the ovum due to concentration abnormalities and sperm motility1.
Acetaminophen, a commonly used analgesic and antipyretic medication, has been reported to have adverse effects on male reproductive health. Prolonged or high-dose exposure to acetaminophen has been associated with altered hormonal balance and reduced semen quality, leading to infertility3,7. There is a need to explore potential therapeutic interventions that can mitigate the negative effects of acetaminophen on male fertility. Avocado leaves have been traditionally used in herbal medicine and have shown promising bioactive properties that may offer protection against reproductive dysfunction8. However, the effects of avocado leaves on the hormonal profile and semen analysis of acetaminophen-induced infertility in male Wistar rats remain largely unexplored3,9,10. It has been suggested that acetaminophen may induce oxidative stress in the testes and other tissues.
Male infertility is a significant health issue affecting couples worldwide, with various factors contributing to its prevalence11. In recent years, a decline in semen quality across Africa, Europe, North America and Asia has been reported3,12. This seems to suggest that male infertility is a growing global problem. Providing a balanced nutritional intake, such as that found in medicinal plants, is one way to try to address infertility8,13. Fruits are one kind of food that supports healthy reproduction.
The avocado fruit (Persea americana) includes protein, vitamins and minerals that can enhance the quality of sperm, making it a food with the potential to improve reproductive health. The pure avocado fruit is packed with healthy ingredients like dietary fiber, potassium, folate, thiamine, riboflavin and extremely high levels of vitamin A, B, C, E and K14. The quantity and quality of sperm can be decreased by the body’s lack of vitamin A, B, C and E15. Men who are infertile can also become fertile again by taking 100 mg/kg of vitamin E daily. This vitamin affects testicular weight, sperm count, motility and estrogen production in addition to boosting sperm survival and development16. In order to produce spermatozoa with appropriate morphology during spermatogenesis in the testes, proteins serve to protect the sperm plasma membrane17. Fatty acids, particularly unsaturated fatty acid groups, are abundant in avocados18. According to Prager et al.19 and Orabueze et al.20, rats fed an avocado oil-based diet for 42 days saw a 4-fold increase in the blood concentration of estradiol and a decrease in testosterone levels, which may boost male fertility.
In Nigeria, male infertility is a significant yet underappreciated reproductive health concern. In certain regions of the nation, male factors account for 20-50% of the causes of infertility. Few researches examined the national reasons for infertility21. Acetaminophen, a widely used medication, has been associated with adverse effects on male reproductive health22. The study evaluated the level of testosterone, Follicle Stimulating Hormone (FSH), Luteinizing (LH) and conduct a seminal fluid assay of a male Wistar rat with acetaminophen-induced infertility and treated with avocado leaves. To evaluate the level of testosterone, FSH and LH, conducted a seminal fluid assay of a male Wistar rat with acetaminophen-induced infertility and not treated with avocado leaves. Ultimately, this study has the potential to improve reproductive health outcomes and offer new avenues for the management of male infertility.
MATERIALS AND METHODS
Collection and authentication of plant material: Ten avocado fruits were procured from botanical garden, at the University of Ibadan, Nigeria. The plant specimen was authenticated by the Department of Zoology. This study was carried out between August, 2023 to November, 2023.
Preparation of plant extract: The leaves were air-dried and 30 g of dried aerial parts of avocado leaves were blended into powder, it was mixed with water (100 mL) and poured inside a bottle, it was stirred and mixed intermittently for 72 hrs. The water extract was filtered using a filter paper into a conical flask and the residue was frozen in a freezer at -84°C. Finally, the filtrate was transferred to a flask and stored until when use (-20°C)23.
Animals acquisition: Thirty adult male Wistar rats weighing 100-130 g were obtained from the animal house, Lead City, University. The rats were housed in plastic mesh cages and maintained in a well-ventilated room at 25±2°C, on a 12 hrs light/12 hrs dark cycle. Rats had unrestricted access to standard rat chow and tap water. The Wistar rats were acclimatized for 2 weeks24.
Ethical consideration: The investigation was conducted in accordance with the National Institutes of Health Guide for the care and use of Laboratory Animals and ethical approval was obtained from the Institutional Review Ethical Committee of Lead City University and the University of Ibadan and every effort was made to minimize both the number of animals used and their suffering.
Experimental grouping and treatment: The rats were divided into 7 groups (6 in each group), the rats were acclimatized for 14 days, then the administration was performed for 14 days. Group 1 was used as a negative control, while group 2 was used as a positive control. Groups of rats were given different treatment regimens.
| Group 1: Rats were given food and water only; it is a negative control | |
| Group 2: Containing acetaminophen (3000 mg/kg) as a positive control | |
| Group 3: Acetaminophen (3000 mg/kg) and standard drugs (silymarin 140 mg) | |
| Group 4: Acetaminophen (3000 mg/kg) and 300 mg/kg Persea americana extract | |
| Group 5: Contains acetaminophen (3000 mg) and 200 mg/kg aqueous extract of Persea americana | |
| Group 6: Acetaminophen (3000 mg/kg) and 100 mg/kg aqueous extract of Persea americana | |
| Group 7: Acetaminophen (3000 mg/kg), standard drugs (silymarin 140 mg) and 100 mg/kg aqueous extracted from the Persea americana |
Sample size: The sample size was determined using Fisher’s formula for cross-sectional study:
Where:
| n | = | Desired sample size (for population greater than 10,000) | |
| Z | = | Standard normal deviation, which is 1.96 (at 95% confidence interval) | |
| p | = | Prevalence of the problem |
Data indicates the global prevalence of male infertility as 2.5 to 12% with 2.5-4.8% infertile men in Sub-Saharan Africa25:
| p | = | 2.8% | |
| q | = | 1-p | |
| q | = | 1-0.028 | |
| d | = | 0.05 (the precision) |
Sample preparation: At the end of treatment (2 months), the rats were anesthetized with pentobarbital sodium (50 mg/kg i.p.). Blood samples were collected from the apex of the heart of the 42 rats into the heparinized bottle and centrifuged at 3000 rpm for 15 min using a bench centrifuge (ERBA) and the plasma will be stored frozen until it is needed for biochemical assay.
Laboratory observation note: The 42 rats were bought from Mr. Gbenga animal house, in which 7 groups were bought and cleaned. Sawdust was placed on the floor of the cage, rat fed was bought and the rats were made comfortable. They were acclimatized for two weeks, to make them adapt to the new environment, however, they were weighed and divided based on their body weight into six groups each in which they were six rats in each cage and were given just food and water. By the 3rd week, the experiment began.
The cages were cleaned and their sawdust was changed every morning. The first group is the control that were given just food and water only for the whole experiment. The 2nd group took 0.5 mL of acetaminophen then with food and water, the 3rd group took 0.5 mL of acetaminophen and 0.5 mL of standard (sylimarin), the 4th group took 0.5 mL of acetaminophen and 0.4 mL of avocado extract, the 5th group took 0.5 mL of acetaminophen and 0.8 mL of avocado extract, the 6th group took 0.5 mL of acetaminophen and the 7th group took 1.5 mL of avocado extract and 0.5 mL of acetaminophen and 0.4 mL of avocado extract and 0.5 mL of standard. They were fed with the aid of gavage.
On observation, group 6 and 7 were observed to be acting reserved, they hardly played nor jumped, then group 4 and 5 were moderately active, they also played with each other and looked like they were fighting.
Group 1, 2 and 3 were super active and also played with each other, but the most active were group 1 and 2. One rat in group 4 died. Whenever they perceive and take the avocado leave extract, they tend to poop more often, both groups 6 and 7 do this the most. However, they prefer to sleep in their food tray and sleep on each other.
Laboratory analysis
Analysis of hormonal profile: Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and testosterone (TT) were measured using enzyme-linked immunosorbent assay kits26 (Elabscience Houston, Texas, 77079, USA).
Principle: The principle of ELISA is based on the specificity of the antigen-antibody interaction. In this technique, a known amount of antigen is immobilized on a solid surface, such as a microtiter plate (10.4" capacitive touch screen, high sensitivity) by Diatek Wuxi City, Jiangsu Province, China. The serum sample containing the target antibody is added to the well and allowed to incubate. If the antigen is present in the serum, it will bind to the immobilized antigen, forming an antigen-antibody complex. After washing, a secondary antibody conjugated with an enzyme is added to the well. This secondary antibody recognizes the primary antibody and binds to it, forming a sandwich-like structure. The excess unbound conjugate is washed off and a substrate solution is added to the well. The enzyme in the conjugate catalyzes a color change in the substrate, which can be measured using a spectrophotometer (ERBA).
Procedures: The FSH, LH and TT-coated microtiter plates were purchased from a commercial source (St Kenny Ventures) and the required reagents were prepared according to the manufacturer’s instructions. The wells of the microtiter plate were coated with the FSH, LH and TT antigen by incubating the plate with a known concentration of FSH, LH and TT solution for 1-2 hrs at room temperature or overnight at 4°C. A known volume of patient serum was added to the well and incubated for 1-2 hrs at room temperature or overnight at 4°C. An enzyme-labeled anti-human IgG antibody was added to the well and incubated for 1 hr at room temperature. A substrate solution (Tetramethylbenzidine (TMB)) was added to the well and incubated for 10-30 min at room temperature. The enzyme in the conjugate catalyzes a color change in the substrate, which was measured using a spectrophotometer. The absorbance values were measured at 450 nm using a spectrophotometer and the results were compared to a standard curve generated using known concentrations of FSH, LH and TT27,28.
Semen analysis
Semen collection: The animals were humanely sacrifices after which a caudoventral mid-abdominal incision was made using a sterilized scissor in order to access the internal organs. The testes were located once pushed upward from the scrotum. Then, the testes were detached from the epididymis and harvested using the method described by Abdul Ganiyu et al.29.
Sperm motility: A drop of semen was placed on a warm microscopic slide mixed with a drop of sodium citrate and covered with a cover slip. The sample was observed under a microscope (Widaco) at X10 magnification and the percentages were recorded; only sperm cells moving in a unidirectional motion were included in the count, while cells moving in circles, backward direction, or pendulous movements were excluded29.
Sperm morphology: Determination of sperm morphology comprises the following:
Preparing a smear of semen on a slide:
| • | Both surfaces of the frosted slides were cleaned by rubbing vigorously with lint-free tissue paper | |
| • | Frosted portion of the slide was labelled with the identifying information (e.g., identification number, date) using a pencil with medium-hard lead | |
| • | A 5-10 μL aliquot of semen was applied, depending on sperm concentration, to the end of the slide, Use a second slide to pull the drop of semen along the surface of the slide | |
| • | Slides were allowed to air dry and then stained5 |
Staining of slide: This was done by using papanicolaou staining technique:
| • | Fixed the air-dried semen smear by immersing slides in 95% (v/v) ethanol for at least 15 min5 | |
| • | Sequentially immerse the slides in: | |
| • | Ethanol 80% (v/v) for 30 sec | |
| • | Ethanol 50% (v/v) for 30 sec | |
| • | Distilled water for 30 sec | |
| • | Harris’s haematoxylin for 4 min | |
| • | Distilled water for 30 sec | |
| • | Acidic ethanol for 4-8 dips* | |
| • | Running cold tap water for 5 min | |
| • | Ethanol 50% (v/v) for 30 sec | |
| • | Ethanol 80% (v/v) for 30 sec | |
| • | Ethanol 95% (v/v) for at least 15 min | |
| • | G-6 orange stain for 1 min | |
| • | Ethanol 95% (v/v) for 30 sec | |
| • | EA-50 green stain for 1 min | |
| • | 2 changes of ethanol 95% (v/v) for 30 sec | |
| • | 2 changes of ethanol 100% for 15 sec | |
| • | Mounted the stained semen smears: | |
| • | Two or three small drops of mounting medium were added to the slide | |
| • | A coverslip (24×50 mm or 24×60 mm are most convenient) was placed directly on the smear | |
| • | Coverslip was positioned so that contact with the mounting medium begins from one long side, to prevent air bubbles from being trapped | |
| • | Excess xylene (if used) was wiped off from underneath the slide | |
| • | Mounted smear was allowed to dry horizontally in a slide drying rack or on absorbent paper for 24 hrs in a fume cupboard5 | |
| • | Viewing: | |
| • | Examined the slide with bright field optics at X1000 magnification with oil | |
| • | Immerse and assessed approximately 200 spermatozoa per replicate for the percentage of normal forms or of normal and abnormal forms5 |
Sperm count: The concentration was determined by the use of the improved Neubauer hemocytometer by Kyrios-Soter Scientific (China). Semen was pipetted to the 0.5 mark using the blood cell pipette and this was made up to the 1.0 mark with normal saline. The normal saline serves both to dilute the semen and fix the spermatozoa present. The pipette was introduced into a pipette shaker and allowed to mix. About 2 or 3 drops of diluted sperm were discarded from the pipette before being introduced under the cover slip on the counting chamber from each side of the hemocytometer. The hemocytometer was carefully placed in a closed pre-wetted chamber for 5 min before being viewed under a light microscope by Omax (Germany) at X40 objective. Sperm heads that have more than half the sperm head within the large five squares that formed the diagonal segment of squares of the hemocytometer chambers were counted. The sperm concentration was determined and calculated as:
Method of data analysis: The primary data was derived from animal studies and presented using simple frequency and percentage tables with explicit narration underneath each table, representing the result derived from the experiment. The statistical software package that was considered for this study is SPSS. The Statistical Package for the Social Sciences (SPSS-IBM, 2020) was used. The mean and standard error of mean (SEM) were calculated for all values. Comparisons between the control and the treated groups were done using the student’s t-test. The differences were considered statistically significant at p<0.05.
RESULTS
The result of the experimental study was presented in tables and charts. The animals were in 7 groups, group 1 was fed with feed and water, group 2 as the positive control was induced with acetaminophen (300 mg/b.wt.), group 3 was fed with acetaminophen (300 mg/b.wt.) and also given silymarin, group 4 was induced with acetaminophen and half dose of extract, group 5 was given acetaminophen and half of the avocado extract in a standard dose, group 6 was given acetaminophen and double dose avocado’s extract while group 7 was given acetaminophen, standard dose and avocado’s extract.
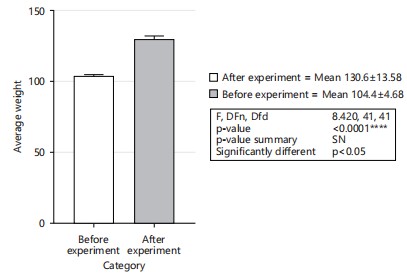
Figure 1 showed the result of the animal body weight. There was no significant difference in the body weight of all the animals across all groups (p = >0.05; F = 6, 30). All the animals were first fed the same way for the 2 week acclimation period. Figure 2 showed the result of the animal’s final body weight. There was significant difference in the body weight of all the animals across all groups in comparison to the initial weight (p = 0.001; F = 8.42) there was an increase in all the groups compared to the initial weight before the experiment. Comparing the weight among the group, there is no significant difference among the group of rats weighed after the experiment p>0.05 (p = 0.493, F = 0.920).
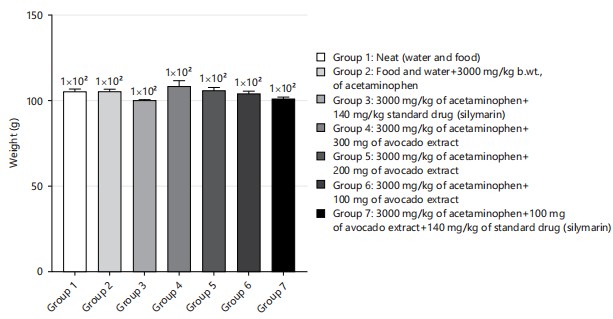
|
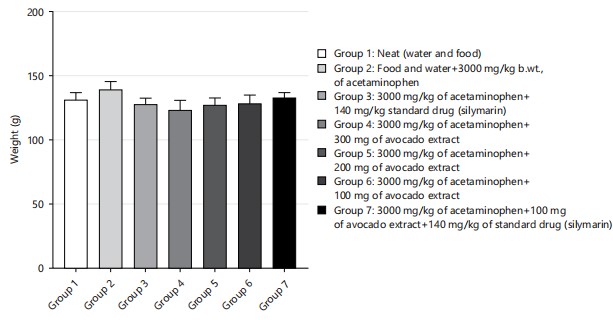
|
However, comparing the initial weight with the final weight, there is a significant difference (F = 8.42, p<0.001). It is found that the weight of the rat increased after the experiment (Fig. 3).
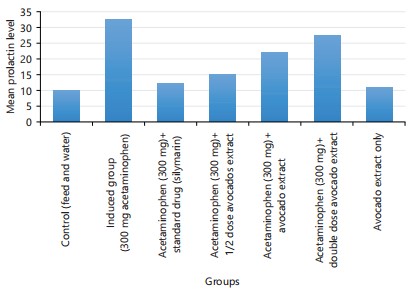
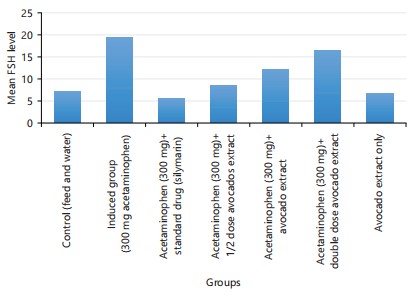
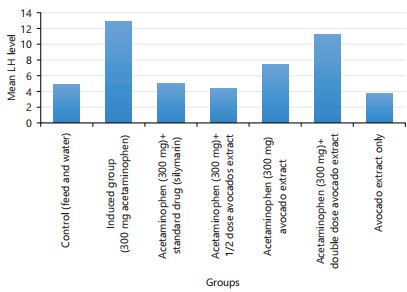
Table 1 showed the descriptive details of hormones (testosterone, prolactin, Follicle Stimulating Hormone, (FSH) and Luteinizing Hormones (LH)). The descriptive details were given by Mean±Standard Deviation. Analysis of variance was performed on the data, there are significant differences across the groups of the experimental rats (p<0.001). Group 2 has the highest mean value in all the hormonal parameters (i.e., group 2 parameters were elevated above all other groups). Table 2 showed the comparison of the hormonal parameters value of the experimental group with the negative control (group 1). An unpaired t-test between group 1 and all other groups was performed. This was done to know the effect of the treatment as compared with the no-treatment group. For testosterone, there are significant differences between group 1 and group 2 (p<0.001), group 4 (p<0.014), group 5 (p<0.0001) and group 6 (p<0.0001). For prolactin; there is a significant difference between group 1 and group 2 (p<0.0001), group 4 (p<0.035), group 5 (p<0.0001) and group 6 (p<0.0001). For FSH; there is a significant difference between group 1 and group 2 (p<0.0001), group 5 (p<0.006) and group 6 (p<0.0001). The LH there is a significant difference between group 1 and group 2 (p<0.002) and group 6 (p<0.0001).
|
| Table 1: | Descriptive statistics and analysis of variance for the hormonal parameters across the groups of experimental rats | |||
| Hormones | Group 1 (n = 6) |
Group 2 (n = 6) |
Group 3 (n = 6) |
Group 4 (n = 6) |
Group 5 (n = 6) |
Group 6 (n = 6) |
Group 7 (n = 6) |
f-value | p-value |
| Testosterone | 16.3±3.1 | 45.2±1.5 | 17.5±1.9 | 21.7±1.6 | 32.5±1.9 | 37.5±1.9 | 20.7±2.2 | 173.98 | 0.000* |
| Prolactin | 10.0±3.2 | 32.5±1.9 | 12.3±3.8 | 15.2±3.3 | 22.0±2.6 | 27.5±1.9 | 11.0±4.0 | 49.93 | 0.000* |
| FSH | 7.3±2.7 | 19.5±1.9 | 5.5±2.9 | 8.5±2.9 | 12.1±1.6 | 16.5±1.5 | 6.6±2.6 | 31.44 | 0.000* |
| LH | 4.8±1.8 | 13.0±2.0 | 5.0±2.4 | 4.4±1.4 | 7.4±1.5 | 11.2±1.5 | 3.8±1.9 | 24.56 | 0.000* |
| ±: Mean-Standard Deviation, *Significant difference (p<0.05), FSH: Follicle Stimulating Hormone and LH: Luteinizing Hormone | |||||||||
| Table 2: | Unpaired t-test of the hormonal parameters of group 1 against the other groups | |||
| Hormones | Group | Mean difference | t- value | Df | p-value |
| Testosterone | 2 | -28.8333 | -30.487 | 5 | 0.000* |
| 3 | -1.1667 | -0.67 | 5 | 0.532 | |
| 4 | -5.3333 | -3.73 | 5 | 0.014* | |
| 5 | -16.1667 | -13.857 | 5 | 0.000* | |
| 6 | -21.1667 | -13.057 | 5 | 0.000* | |
| 7 | -4.3333 | -2.335 | 5 | 0.067 | |
| Prolactin | 2 | -22.5 | -14.375 | 5 | 0.000* |
| 3 | -2.3333 | -1.282 | 5 | 0.256 | |
| 4 | -5.1667 | -2.876 | 5 | 0.035* | |
| 5 | -12 | -12 | 5 | 0.000* | |
| 6 | -17.5 | -12.426 | 5 | 0.000* | |
| 7 | -1 | -0.466 | 5 | 0.661 | |
| FSH | 2 | -12.2333 | -8.315 | 5 | 0.000* |
| 3 | 1.8167 | 0.974 | 5 | 0.375 | |
| 4 | -1.2 | -0.804 | 5 | 0.458 | |
| 5 | -4.85 | -4.637 | 5 | 0.006* | |
| 6 | -9.2667 | -8.134 | 5 | 0.000* | |
| 7 | 0.65 | 0.458 | 5 | 0.666 | |
| LH | 2 | -8.1333 | -6.273 | 5 | 0.002* |
| 3 | -0.2167 | -0.15 | 5 | 0.887 | |
| 4 | 0.4 | 0.482 | 5 | 0.65 | |
| 5 | -2.5833 | -2.55 | 5 | 0.051 | |
| 6 | -6.4167 | -9.562 | 5 | 0.000* | |
| 7 | 1.0333 | 1.494 | 5 | 0.195 | |
| *Significant difference (p<0.05), FSH: Follicle Stimulating Hormone and LH: Luteinizing Hormone | |||||
|
| Table 3: | Unpaired t-test of the hormonal parameters of group 2 (Induced group) against the other groups | |||
| Hormones | Group | Mean difference | t-value | Df | p-value |
| Testosterone | 1 | 28.8333 | 30.487 | 5 | 0 |
| 3 | 27.6667 | 26.247 | 5 | 0 | |
| 4 | 23.5 | 22.239 | 5 | 0 | |
| 5 | 12.6667 | 12.017 | 5 | 0 | |
| 6 | 7.6667 | 7.064 | 5 | 0.001 | |
| 7 | 24.5 | 16.84 | 5 | 0 | |
| Prolactin | 1 | 22.5 | 14.375 | 5 | 0 |
| 3 | 20.1667 | 10.049 | 5 | 0 | |
| 4 | 17.3333 | 17.529 | 5 | 0 | |
| 5 | 10.5 | 9.151 | 5 | 0 | |
| 6 | 5 | 4.038 | 5 | 0.01 | |
| 7 | 21.5 | 10.597 | 5 | 0 | |
| FSH | 1 | 12.2333 | 8.315 | 5 | 0 |
| 3 | 14.05 | 7.872 | 5 | 0.001 | |
| 4 | 11.0333 | 13.343 | 5 | 0 | |
| 5 | 7.3833 | 8.554 | 5 | 0 | |
| 6 | 2.9667 | 3.027 | 5 | 0.029 | |
| 7 | 12.8833 | 17.484 | 5 | 0 | |
| LH | 1 | 8.1333 | 6.273 | 5 | 0.002 |
| 3 | 7.9167 | 5.656 | 5 | 0.002 | |
| 4 | 8.5333 | 13.68 | 5 | 0 | |
| 5 | 5.55 | 4.701 | 5 | 0.005 | |
| 6 | 1.7167 | 1.726 | 5 | 0.145 | |
| 7 | 9.1667 | 10.063 | 5 | 0 |
Table 3 showed the comparison of the hormonal parameters value of the experimental group with the positive control (group 2). An unpaired t-test between group 2 and all other groups was performed. This was done to know the effect of overdosed acetaminophen as compared with the treatment method specified in other groups. There is a significant difference between group 2 and other groups in all the parameters (p<0.05).
|
|
Figure 4 to 7 shows the graphical representation of the mean value of each parameter across the 7 groups.
Semen analysis: Calculating the SEM analysis, there is a significant variation in group 2 compared to the other group. Meanwhile, other groups that involves in the treatment group exhibit variation too but not to a significant level (Table 4).
DISCUSSION
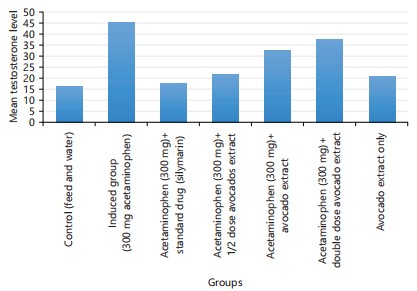
Avocado leaf effects observed highly significant increase in values of testosterone (ng/mL) in all concentrations of avocado leaf extract (group 4, 5 and 6) when compared with negative control groups (group 1). When compared with control groups, highly significant increase (p<0.001) in testosterone level was illustrated in experimental treated groups with concentrations of 3000 mg/kg of acetaminophen (group 2). However, male infertility is intended as a condition for men who are unable to impregnate their partners for at least one year after intercourse without using a protector1. Low sperm count or an imbalance in hormones could be the cause of this. The gonads are the primary site of production for sex hormones, especially testosterone in males, where FSH and LH have an influence. It is well recognized that elevated levels of sex hormones have a positive feedback effect at the pituitary gland level, where they control gonadotropin output.
|
| Table 4: | Description of the semen obtained from the experimental rats | |||
| Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
| pH | Alkaline | Alkaline | Alkaline | Alkaline | Alkaline | Alkaline | Alkaline |
| Consistency | Moderately | Highly | Moderately | Moderately | Moderately | Moderately | Moderately |
| visious | visious | visious | visious | visious | visious | visious | |
| Colour | Grayish | Grayish | Grayish | Grayish | Grayish | Grayish | Grayish |
| Total count6 | 55×106 sperm/cell |
10×106 sperm/cell |
95×106 sperm/cell |
- | 60×106 sperm/cell |
12×106 sperm/cell |
96×106 sperm/cell |
| Motility | |||||||
| Actively motile | 80 | 12 | 82 | 77 | 60 | 43 | 90 |
| Sluggishly motile | 10 | 58 | 12 | 10 | 25 | 28 | 5 |
| Dead/immotile | 10 | 30 | 6 | 13 | 15 | 29 | 5 |
| Morphology | |||||||
| Normal shape | 80 | 10 | 76 | 80 | 64 | 40 | 88 |
| Abnormal shape | 15 | 70 | 10 | 12 | 26 | 44 | 10 |
| Partial shape | 5 | 20 | 14 | 8 | 10 | 16 | 2 |
The study discovered that giving avocado (Persea americana) leaf extract to male rats raised their blood levels of testosterone, FSH, LH and prolactin. In the current investigation, however, there was a marginally significant difference in the serum levels of LH, FSH and prolactin between the rats in the silymarin and Persea americana leaf extract-treated groups and the rats in the control group. Based on the study’s findings, it appears that silymarin and P. americana leaf extract primarily benefit the gonads, promoting the release of these gonadal hormones into the bloodstream in men. Rats given acetaminophen (group 2) were found to have a greater overall hormone secretion stimulating effect than rats given P. americana extract (group 4, 5, 6 and 7).
The results of this present study revealed that som e of the chemical agent(s) which are contained in P. americana leaf extracts possess stimulatory activity on the reproductive functions in rats model. Serum concentration of FSH significantly increased in a dose-dependent manner following treatment with P. americana in all test groups when compared with control (p<0.05) but fell when given acetaminophen+standard drugs (silymarin) and aqueous extract from the Persea americana (group 7). Testosterone concentrations in all test groups were considerably greater when compared with those of the control group.
The extract may have enhanced the male rats’ reproductive activity, as evidenced by the rise in Follicle Stimulating Hormone (FSH) concentration after treatment. In men, free sperm production is stimulated and maintained to a normal degree, which in turn controls sexual development, growth, pubertal maturation and reproductive capabilities30. However, because acetaminophen induces spermatogenesis, an excessive rise in FSH levels, as seen in rats given 3000 mg/kg of the drug, may have a deleterious effect on reproduction. It has been determined that high FSH concentration, which typically denotes a main testicular malfunction and spermatogenesis impairment, is the most prevalent endocrine problem linked to male infertility or subfertility31.
The administration of high-dosage extract may be harmful to the male reproductive system, as evidenced by the fact that the concentrations of prolactin and testosterone hormones rose in the rats treated with lower doses but decreased in the group given a higher dose. Prolactin hormone may be the cause of the rise in testosterone concentration in the low and moderate dose treated groups. Prolactin hormone stimulates testosterone production from Leydig cells in the testis, which increases male reproductive functions31. In males, testosterone, a primary sex hormone, stimulates the growth of the reproductive organs and secondary sexual traits31. The observed reduction in testosterone concentration after high-dose treatment could have been caused by the extract’s direct impact on the Leydig cells or indirectly by the group’s lower prolactin hormone levels. A significant indicator of infertility is said to be a decline in testosterone concentration32.
The color of the sperm samples did not substantially change between the test groups and the control, indicating that the extract treatment had no effect on their expectedly creamy hue, which is associated with improved reproductive performance. Sperm sample pH is a well-known indicator of the survival rate of sperm cells and a crucial parameter in the assessment of sperm sample quality. Acidic environments are known to raise sperm mortality, impair sperm function and conception and contribute to infertility33. This can occur in the female vagina or in the sperm sample itself. Because alkaline media neutralizes the acidity of the female vagina and creates an environment that maximizes sperm motility and improves the processes leading to ovum fertilization, it is therefore the most optimal medium for sperm performance31. Therefore, although the low pH seen in all groups may correlate with an inhibitory effect on both sperm survival and reproduction, the alkaline semen environment identified in all groups implies that the dose favors reproduction in rats. Notably, individual sperm motility, progressiveness and viability in this therapy group were all identical to those in the control group. Progression in sperm motility has been associated with a high fertility index, according to Love34. The fructose content of sperm samples has been shown to increase sperm motility; however, at the greatest dose, the extract may have decreased the fructose content of the sperm sample, resulting in a decrease in sperm motility and viability. These results seem to corroborate a research which showed that large dosages of Persea americana extract had detrimental effects on male rats’ reproductive systems35.
CONCLUSION
The study revealed positive changes in hormonal profiles and semen analysis parameters, suggesting a potential protective role of avocado leaves against the detrimental impacts of acetaminophen on reproductive health. In view of the pending observation of the effectiveness of the leave Persea americana and the ability to reverse the damage caused by acetaminophen, it is recommended that Persea americana leave should be incorporated into spectrum of herbal drugs for the management of hormonal imbalance.
SIGNIFICANCE STATEMENT
This study aims to investigate and evaluate the effects of avocado leaves on hormones and semen of acetaminophen-induced infertility in male Wistar rats and to conduct a seminal fluid assay on the rats treated with avocado leaves. The avocado extract reduces body weight by a significant level. Excessive increase in FSH concentration as observed in the 3000 mg/kg of acetaminophen-treated rats may negatively affect reproduction due to its effect on spermatogenesis. As observed, testosterone in the lower doses increased but decreased in the high dose group. It is recommended that Persea americana leaves should be incorporated into a spectrum of herbal drugs for the management of hormonal imbalance, however, high doses should not be taken into consideration to prevent oligospermia and azoospermia.
ACKNOWLEDGMENT
The authors would like to acknowledge the management and all the technical staff of St Kenny Research Consult, Ekpoma, Edo State, Nigeria for their excellent assistance and for providing medical writing/editorial support in accordance with Good Publication Practice (GPP3) guidelines.
REFERENCES
- Amelia, Y.T., E. Hanizar and D.N.R. Sari, 2021. The effect of consuming avocado (Persea americana) on mice (Mus musculus) sperm quality. BIOVALENTIA: Biol. Res. J., 7: 11-17.
- Agarwal, A., N. Parekh, M.K.P. Selvam, R. Henkel and R. Shah et al., 2019. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Mens Health, 37: 296-312.
- Momoh, A.R.M., P.O. Orhue, P.O. Okolo, D. Odaro, A.A. Momoh and L.K. Iyevhobu, 2012. The antibiogram types of auto-agglutinating Staphylococcus aureus strains isolated from the semen samples of males with infertility problems in Edo State, Nigeria. E3 J. Med. Res., 1: 17-24.
- Yu, G., Z. Bai, C. Song, Q. Cheng, G. Wang, Z. Tang and S. Yang, 2021. Current progress on the effect of mobile phone radiation on sperm quality: An updated systematic review and meta-analysis of human and animal studies. Environ. Pollut., 282.
- Björndahl, L. and J.K. Brown, 2022. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: Ensuring quality and standardization in basic examination of human ejaculates. Fertil. Sterility, 117: 246-251.
- Hosomi, R., M. Yoshida and K. Fukunaga, 2012. Seafood consumption and components for health. Global J. Health Sci., 4: 72-86.
- Alshailabi, E.M., O.A. Abdalally and F.A. Mohammed, 2024. The protective role of ascorbic acid on the testis tissue damage induced by paracetamol in albino rats. Al-Kitab J. Pure Sci., 8: 19-28.
- Iyevhobu, K.O., A.I. Airefetalor, E.K. Osagiede, L.E. Omolumen, R.E. Ikede and B.A. Ken-Iyevhobu, 2022. Effects of some herbal plants and herbal drink on selected micro-organisms using ditch and agar methods. South Asian Res. J. Appl. Med. Sci., 4: 14-19.
- Israr, F., M.T. Javed, M.H. Ahmed, A. Tariq and S. Zarnab et al., 2022. Effect of different doses of acetaminophen through drinking water on body organs and serum biochemical parameters in broilers. Toxin Rev., 41: 1086-1095.
- Banihani, S.A., 2017. Effect of paracetamol on semen quality. Andrologia, 50.
- Lee, Y.C., Y.T. Su, T.Y. Liu, C.M. Tsai, C.H. Chang and H.R. Yu, 2018. L-arginine and L-citrulline supplementation have different programming effect on regulatory T-cells function of infantile rats. Front. Immunol., 9.
- Dissanayake, D.M.I.H., W.L.R. Keerthirathna and L.D.C. Peiris, 2019. Male infertility problem: A contemporary review on present status and future perspective. Gender Genome, 3.
- Kusuma, S., A. Setiawan and S. Salni, 2018. The capability of sedative effect from celery (Apium graveolens L.) fraction to male mice. BIOVALENTIA: Biol. Res. J., 4: 22-27.
- Nair, S.S. and C.C. Anitha, 2018. Nutrient composition of avocado fruits of selected cultivars grown in Kerala. Int. J. Food Sci. Nutr., 3: 65-67.
- Dreher, M.L. and A.J. Davenport, 2013. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr., 53: 738-750.
- Shehata, M.M.S.M. and S.S.A. Soltan, 2013. Effects of bioactive component of kiwi fruit and avocado (fruit and seed) on hypercholesterolemic rats. World J. Dairy Food Sci., 8: 82-93.
- Biswas, S. and P.K. Mukhopadhyay, 2020. Casein- and pea-enriched high-protein diet can take care of the reprotoxic effects of arsenic in male rats. Andrologia, 52.
- Martínez-Soto, J.C., J.C. Domingo, B. Cordobilla, M. Nicolás and L. Fernández et al., 2016. Dietary supplementation with Docosahexaenoic Acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med., 62: 387-395.
- Prager, N., K. Bickett, N. French and G. Marcovici, 2006. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-α-reductase in the treatment of androgenetic alopecia. J. Altern. Complementary Med., 8: 143-152.
- Orabueze, I.C., R. Babalola, O. Azuonwu, I.I. Okoko and G. Asare, 2021. Evaluation of possible effects of Persea americana seeds on female reproductive hormonal and toxicity profile. J. Ethnopharmacol., 273.
- Emokpae, M.A. and S.I. Brown, 2021. Effects of lifestyle factors on fertility: Practical recommendations for modification. Reprod. Fertil., 2: R13-R26.
- Alchin, J., A. Dhar, K. Siddiqui and P.J. Christo, 2022. Why paracetamol (acetaminophen) is a suitable first choice for treating mild to moderate acute pain in adults with liver, kidney or cardiovascular disease, gastrointestinal disorders, asthma, or who are older. Curr. Med. Res. Opin., 38: 811-825.
- Kose, L.P., Z. Bingol, R. Kaya, A.C. Goren and H. Akincioglu et al., 2020. Anticholinergic and antioxidant activities of avocado (Folium perseae) leaves- phytochemical content by LC-MS/MS analysis. Int. J. Food Prop., 23: 878-893.
- Brai, B.I.C., J.A. Falode, R.A. Adisa and A.A. Odetola, 2020. Effects of aqueous leaf extract of avocado (Persea americana) on total cholesterol, triacylglycerols, protein and haematological parameters in CCl4-intoxicated rats. Clin. Phytosci., 6.
- Ikyernum, J.A., A. Agbecha and S.T. Hwande, 2019. Semen profile of men presenting with infertility at first fertility hospital Makurdi, North Central Nigeria. Clin. Med. Diagn., 9: 26-35.
- Książek, A., M. Mędraś, A. Zagrodna, M. Słowińska-Lisowska and F. Lwow, 2021. Correlative studies on vitamin D and total, free bioavailable testosterone levels in young, healthy men. Sci. Rep., 11.
- Mir, M.A., S. Aisha, S. Nisar, H. Qayoom and U. Mehraj, 2022. Immuno-Onco-Metabolism and Therapeutic Resistance. In: Immuno-Oncology Crosstalk and Metabolism, Macha, M.A., A.A. Bhat and N.A. Wani, (Eds.), Springer, Singapore, ISBN: 978-981-16-6226-3, pp: 45-89.
- Fang, L., Y. Ma, Y. Peng, J. Ni and C. Ma et al., 2024. Long-term effect of fine particulate matter constituents on reproductive hormones homeostasis in women attending assisted reproductive technologies: A population-based longitudinal study. Ecotoxicol. Environ. Saf., 284.
- Abdul Ganiyu, L., A.T. Wahab and M.O.B. Oyeyemi, 2022. Fertility assessment of the male albino rats (Wistar strain) treated with aqueous and ethanol leaf extracts of Euphorbia hirta Linn. Acta Sci. Vet. Sci., 4: 63-73.
- Vasudevan, D.M., S. Sreekumari and K. Vaidyanathan, 2011. Textbook of Biochemistry for Medical Students. 6th Edn., Jaypee Brothers Medical Publishers, New Delhi, India, ISBN: 9789350250167, Pages: 657.
- Kobayashi, H., K. Nagao and K. Nakajima, 2012. Focus issue on male infertility. Adv. Urol., 2012.
- Okereke, C. and S. Onuoha, 2015. Effect of ethanolic extract of Cannabis sativa on progesterone and estrogen hormones in female Wistar rats. Reprod. Syst. Sexual Disord.: Curr. Res., 4.
- Zhou, J., L. Chen, J. Li, H. Li and Z. Hong et al., 2015. The semen pH affects sperm motility and capacitation. PLoS ONE, 10.
- Love, C.C., 2011. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology, 76: 547-557.
- Ugwu, N.I., C.L. Uche, A.I. Airaodion, A.A. Ogbenna and K. Chikezie et al., 2024. Impact of Corchorus olitorius leaf extract on potassium bromate-induced haematological parameters derangement in rats. Trop. J. Nat. Prod. Res., 8: 7786-7792.
How to Cite this paper?
APA-7 Style
Stanley,
U.O., Oshiokhayamhe,
I.K., Oberhiri,
O.K., Eromosele,
O.L., Osayande,
A.K., Ernest,
A., Osaze,
I.I., Moses,
M.J., Junior,
A.A., Ehizojie,
I.R., Emmanuel,
O.O., Jesse,
O., Bolade,
L.S., Abimbola,
A.A. (2025). Acetaminophen-Induced Infertility in Male Wistar Rats: Impact of Avocado Leaves on Hormonal Profile and Semen Analysis. Asian Journal of Biological Sciences, 18(1), 68-82. https://doi.org/10.3923/ajbs.2025.68.82
ACS Style
Stanley,
U.O.; Oshiokhayamhe,
I.K.; Oberhiri,
O.K.; Eromosele,
O.L.; Osayande,
A.K.; Ernest,
A.; Osaze,
I.I.; Moses,
M.J.; Junior,
A.A.; Ehizojie,
I.R.; Emmanuel,
O.O.; Jesse,
O.; Bolade,
L.S.; Abimbola,
A.A. Acetaminophen-Induced Infertility in Male Wistar Rats: Impact of Avocado Leaves on Hormonal Profile and Semen Analysis. Asian J. Biol. Sci 2025, 18, 68-82. https://doi.org/10.3923/ajbs.2025.68.82
AMA Style
Stanley
UO, Oshiokhayamhe
IK, Oberhiri
OK, Eromosele
OL, Osayande
AK, Ernest
A, Osaze
II, Moses
MJ, Junior
AA, Ehizojie
IR, Emmanuel
OO, Jesse
O, Bolade
LS, Abimbola
AA. Acetaminophen-Induced Infertility in Male Wistar Rats: Impact of Avocado Leaves on Hormonal Profile and Semen Analysis. Asian Journal of Biological Sciences. 2025; 18(1): 68-82. https://doi.org/10.3923/ajbs.2025.68.82
Chicago/Turabian Style
Stanley, Usiobeigbe, Osahon, Iyevhobu Kenneth Oshiokhayamhe, Obohwemu Kennedy Oberhiri, Omolumen Lucky Eromosele, Airhomwanbor Kingsley Osayande, Asibor Ernest, Irobonosen Israel Osaze, Malgwi Jemimah Moses, Adeji Anthony Junior, Ikede Rex Ehizojie, Omoviye Onomen Emmanuel, Omoregie Jesse, Lagundoye Sherifat Bolade, and Adelani Aderonke Abimbola.
2025. "Acetaminophen-Induced Infertility in Male Wistar Rats: Impact of Avocado Leaves on Hormonal Profile and Semen Analysis" Asian Journal of Biological Sciences 18, no. 1: 68-82. https://doi.org/10.3923/ajbs.2025.68.82

This work is licensed under a Creative Commons Attribution 4.0 International License.



