Monitoring of Low-Dose Effects of Bisphenol a on Superoxide Dismutase and Catalase Level in Female Rats
| Received 20 Jul, 2022 |
Accepted 05 Sep, 2022 |
Published 31 Mar, 2023 |
Background and Objective: Inflammatory diseases are becoming increasingly prevalent worldwide, Exposure to environmental pollutants such as BPA could be one of the risk factors responsible for the development of such diseases. This study investigated the relationships between the serum levels of two endogenous antioxidants Enzyme and Bisphenol A concentrations. Materials and Methods: The study groups were divided into eleven healthy experimental animals. Serum Superoxide Dismutase (SOD) and Catalase (antioxidant enzymes), profiles were analyzed by spectrophotometric methods. Results: Serum Superoxide Dismutase (SOD) and Catalase (CAT) levels were significantly decreased in treated groups compared with control (p<0.05). Conclusion: This study demonstrated that the dose of BPA not only increases the free radical formation but also decreases its ability to detoxify ROS.
| Copyright © 2023 Oguazu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Bisphenol A (BPA) is a monomer used in the production of Polycarbonate plastics, it is also in many consumer products as lacquers applied as food can linings and coating on metal lids for glass jars and bottles1. Polycarbonate plastics are used to make a variety of common products used in food contact materials2. It is used in products like adhesive and flooring materials and paints and varnishes3. The BPA metabolism is dominated by phase II conjugation reaction in the hepato-intestinal tract4 and it is removed from the blood by first-pass metabolism in the liver5. The main route of excretion is via faeces and urine4.
Reports have shown that environmentally relevant doses of BPA can cause effects on human development and reproduction which include but are not limited to increases in weight and size of the prostate gland, decreases in sperm efficiency2 and abnormalities in the oocytes6. Damage to the liver and kidney7,8, DNA adduct formation9, adipose tissue dysfunction10, impaired plasma glucose11 and recurrent miscarriage and birth defects12.
Low doses of BPA inhibit microtubule polymerization, affect the spindle apparatus and produce aneuploidy2, congression failure, chromosomal misalignment and aneuploidy in oocytes13, involved in insulin resistance14, Synaptic abnormalities and recombination aberration in oocytes15, metabolic/ endocrine dysfunctions10, cancer and fertility problems16 and alterations of various brain nuclear receptors, alongside, increased progesterone receptor immunoreactivity17 and enhanced antagonism at thyroid receptors18. The BPA provoked an increase in body weight19, spurring the formation and growth of fat cells17, abnormal levels of the liver enzyme γ-glutamyl-transferases, alkaline phosphatase and lactate dehydrogenase8,20. BPA has adverse effects on testicular function21 and it interferes with LH receptor-ligand binding22. Li et al.23, reported reduced sexual desire, erectile or ejaculation difficulty and reduced sexual satisfaction. Rebai et al.24, reported urinary BPA levels in workers of BPA manufacturing facilities In male species, researchers reported a decrease in steroidogenic enzymes25,26. Yang et al.27, a study showed that circulating levels of inflammation factors were increased in response to BPA exposure. It has been shown that many environmental contaminants can induce oxidative stress, Chou et al.28, showed that BPA can decrease the activity of antioxidant enzymes in the liver. Higher doses of BPA also provoked antioxidant activity29. Rashid et al.30, showed oxidative stress BPA can cause oxidative stress by disturbing the redox status in cells31.
This study aims to unveil/establish the possible effects and physiological disposition of bisphenol A on oxidative stress markers in female Wistar albino rats.
MATERIALS AND METHODS
Study area: The study was carried out at the Department of Biochemistry, Research Laboratory, Faculty of Natural and Applied Sciences, Gregory University, Uturu, Abia State Nigeria, from June to August, 2021.
Sample collection: Total 60 non-pregnant female rats of 5 weeks age were acclimatized in the laboratory for 7 days and randomly divided into 11 experimental groups of 5 rats each and respectively administered, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1 mg of BPA/kg.b.wt./day. The first group which served as control did not receive any treatment but distilled water instead. The graded doses of BPA were dissolved in distilled water and administered by oral gavage using an intubation cannula (Lars Medicare Pvt., Ltd., new Delhi, India). Blood was obtained from the tail of the various groups by capillary action, weekly, after BPA administration for 13 weeks. Blood samples were processed for clinical assay.
Experimentation: Animals have housed in aluminium wire-mesh cages in a well-ventilated animal house with a 12 hrs dark/light cycle and at room temperature and were provided commercial rat pellets (Vital feed from Vital Group of Company, Nigeria) and water ad libitum.
At the end of the experiments, serum catalase and SOD were assayed using an Autochemical Analyzer (Lx 20 pro Autoanalyser, Beckman Coulter, Woerden, Netherland and Chemwell Chemical Analyzer, Manufacturer: Roche Hitachi, GMI.). All reagents were commercially obtained as already prepared kits. The kits for catalase and SOD were purchased from the Abcam United Kingdom. Individual tests were carried out according to the kit specifications.
Statistical analysis: Differences between obtained values (Mean±SD) were carried out by One-way Analysis of Variance (ANOVA) using SPSS software version 20.0 followed by the Tukey-Kramer Multiple Comparison Test. At p≤0.05 was taken as a criterion for a statistically significant difference.
RESULTS
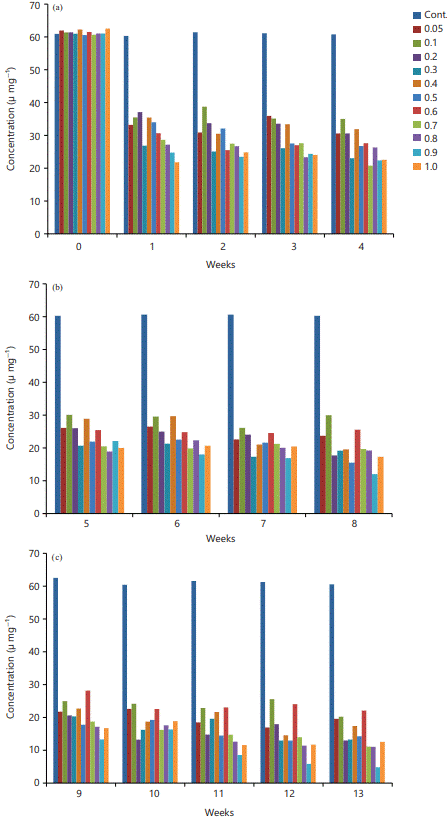
Superioxide Dismutase (SOD): There is a significant decrease in the SOD activity in all the test groups when compared with the control at p≤0.05 (Fig. 1a-c). The activities fluctuate at various points of measurement (Fig. 1a-c). The observed decrease in SOD was consistent throughout the exposure.
|
|
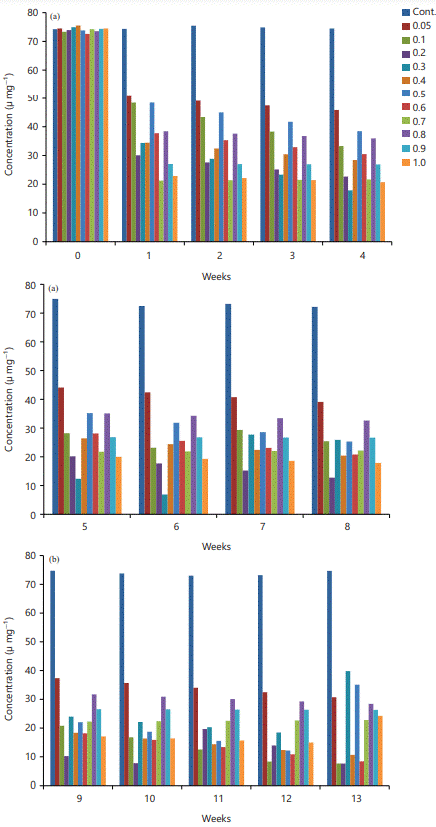
Catalase: There is a significant decrease in the catalase activity in all the BPA-exposed groups when compared with the control p≤0.05 (Fig. 2a-c). The weeks of exposure varied across the groups (Fig. 2a-c). The group that received 0.9 mg kg–1 b.wt., of BPA showed a significant decrease in catalase that remain constant throughout the 13 weeks of exposure (Fig. 2c). Those that received 0.7 mg kg–1 b.wt., of BPA showed a significant decrease in catalase when compared with the control but non-significant variation from the 1st week to the 13th week (Fig. 2a-c). The other experimental groups revealed a significant decrease in catalase but interestingly also the catalase level decreases with an increased period of exposure to BPA (Fig. 2a-b), except for the 13th week of 0.3, 0.5 and 1.0 mg kg–1 b.wt., of BPA Administration, in which the catalase level tends to rise but not above the control (Fig. 2c).
DISCUSSION
The result of this experiment showed that there is a decrease in Superoxide Dismutase (SOD) and catalase. BPA induces Reactive Oxygen Species (ROS) production and significantly compromises mitochondrial function. The decrease in CAT activity increased the toxic effect of the free radicals formed from the BPA effect. Because BPA caused the induction of free radicals in the hepatic tissue, in consequence, it leads to disruption in the antioxidant defence system. It was found that BPA disturbs the balance of the mitochondrial antioxidant-pro oxidant status through the reduction of the activities of mitochondrial respiratory chain enzymes, which may cause mitochondrial dysfunction and increased ROS generation32. Additionally, it could be mediated through the ability of BPA to stimulate the polymorphism of oxidative stress-related genes33. The decrease in activities of the antioxidant enzymes might predispose the liver to increased free radical damage because CAT has been considered the primary scavenger of H2O234, SOD can catalyse the decomposition of superoxide radicals to produce H2O2. However, in absence of adequate CAT activity to degrade H2O2, more H2O2 could be converted to toxic hydroxyl radicals and may contribute to the oxidative stress of BPA, Which indicated liver tissue damage35. A high BPA dose tends to increase the free radical formation and decrease the cell's ability to detoxify reactive oxygen species36. The superoxide radicals and NO formation forms peroxynitrite as a result of high doses of BPA exposure leading to tissue damage and in turn, increasing the levels of NO36,37.
Targets of oxidative stress include phospholipid membranes, proteins and nucleic acids. As such, increased systemic oxidative stress can lead to irreversible changes in these molecules as well as in mitochondria38.
Following our finding, Hassan et al.36, also show a decrease in SOD. Kourouma et al.39, show a decrease in the activities of antioxidant enzymes, namely, CAT and SOD. Wu et al.40, demonstrated significant decrease SOD. Hassan et al.36, showed a decrease in CAT activity. Eid et al.41, also demonstrated a decrease in the activities of antioxidant enzymes SOD and CAT. Aboul Ezz et al.42, revealed a decrease the catalase activity. Abedelhaffeza et al.43, observed decreased SOD activities in BPA administration. Chitra et al.44, showed that the activities of superoxide dismutase and catalase, were decreased.
The reduced activity of catalase is linked to the depletion of the enzyme and enzyme inactivation caused by excess ROS production in mitochondria and microsomes42 after BPA exposure. The observed decreased SOD activities, accompanied by the decreased CAT activities could be because of the metabolism of BPA in the liver, where it is glucuronidated by liver microsomes45, mediated by UGT2B1, an isoform of UGT in rat liver. The metabolites of BPA produced by microsomal Cytochrome P450s enhance estrogenic activity39. Again, the mechanisms by which SOD can lead to increased cell death46 and induced apoptosis is its ability to activate p53 by the production of H2O234 have been reported46. Also, another mechanism for SOD-. The reduction in the activity of catalase may be due to the exhaustion of the enzyme in attempting to eliminate the hydrogen peroxide generated after exposure to BPA. This may also be due to enzyme inactivation caused by excess ROS production in mitochondria and microsomes47. It was found that BPA disturbs the balance of the mitochondrial antioxidant-pro oxidant status through the reduction of the activities of mitochondrial respiratory chain enzymes, which may cause mitochondrial dysfunction and increased ROS generation32. Additionally, it could be mediated through the ability of BPA to stimulate the polymorphism of oxidative stress-related genes33.
This research implies that regardless of the presence of this antioxidant system, an over or unbalanced production of ROS due to contact with the chemical may result in several clinical disorders. With the growing epidemic of disease worldwide and the extensive use of consumer goods containing BPA, the risk of BPA as a potential triggering compound in disease must be examined.
CONCLUSION
There is increasing evidence that BPA is a toxic compound. However, the degree of toxicity depends on the dose, time, frequency, individual differences and age of exposure. The BPA is an endocrine disorderly chemical released in the environment, so most studies are focused on its effect on reproduction. Antioxidants reduce the cellular damage resulting from the interaction between lipid, protein and DNA molecules and ROS. Regardless of the presence of this antioxidant system, an over or unbalanced production of ROS due to contact with the chemical may result in several clinical disorders. With the growing epidemic of disease worldwide and the extensive use of consumer goods containing BPA, the risk of BPA as a potential triggering compound in disease must be examined. Many of the mechanisms known to exist in disease pathophysiology also appear to exist with immune reactivity from BPA exposure. In addition, severe oxidative stress resulting from early life exposure to BPA could lead to DNA damage and mutation of tumour suppressor genes. The cells have various defense mechanisms against oxidative stress, including enzymatic scavengers; that protect the system from the deleterious effects of ROS. The study revealed that BPA caused marked oxidative impact by decreasing the activities of antioxidant enzymes compared to their activities in the control group. This study demonstrated that a dose of BPA not only increases the free radical formation but also decreases its ability to detoxify ROS.
REFERENCES
- Chapin, R.E., J. Adams, K. Boekelheide, L.E. Gray Jr. and S.W. Hayward et al., 2008. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. Part B: Dev. Reprod. Toxicol., 83: 157-395.
- Christiansen, S., M. Axelstad, J. Boberg, A.M. Vinggaard, G.A. Pedersen and U. Hass, 2014. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction, 147: 477-487.
- Vogel, S.A., 2009. The politics of plastics: The making and unmaking of bisphenol A “safety”. Am. J. Public Health, 99: S559-S566.
- Rodriguez-Hernandez, H., L.E. Simental-Mendia, G. Rodriguez-Ramirez and M.A. Reyes-Romero, 2013. Obesity and inflammation: Epidemiology, risk factors and markers of inflammation. Int. J. Endocrinol.
- Durcik, M., D.G. Skledar, T. Tomašič, J. Trontelj and L.P. Mašič, 2022. Last piece in the puzzle of bisphenols BPA, BPS and BPF metabolism: Kinetics of the in vitro sulfation reaction. Chemosphere.
- Kawato, S., M. Ogiue-Ikeda, M. Soma, H. Yoshino and T. Kominami et al., 2021. Perinatal exposure of bisphenol A differently affects dendritic spines of male and female grown-up adult hippocampal neurons. Front. Neurosci.
- Ezeonu, F.C., C.E. Oguazu, K.I. Ubaoji and B. Anajekwu, 2015. Bisphenol A causes blood electrolyte imbalance and upsets kidney functions in albino Wistar rats. J. Pharm. Sci. Bioscientific Res., 5: 547-550.
- Oguazu, C.E., F.C. Ezeonu, K.I. Ubaoji and B. Anajekwu, 2015. Bisphenol A exerts a transient perturbation of liver function in Wistar albino rats at acute and sub-chronic exposure doses. J. Pharm. Sci. Bioscientific Res., 5: 274-278.
- LaKind, J.S., M. Goodman and D.Q. Naiman, 2012. Use of NHANES data to link chemical exposures to chronic diseases: A cautionary tale. PLoS ONE.
- Shankar, A. and S. Teppala, 2011. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab., 96: 3822-3826.
- Teppala, S., S. Madhavan and A. Shankar, 2012. Bisphenol A and metabolic syndrome: Results from NHANES. Int. J. Endocrinol.
- Khan, N.G., J. Correia, D. Adiga, P.S. Rai and H.S. Dsouza et al., 2021. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidence. Environ. Sci. Pollut. Res., 28: 19643-19663.
- Hunt, P.A., K.E. Koehler, M. Susiarjo, C.A. Hodges and A. Ilagan et al., 2003. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Current Biol., 7: 546-553.
- Gerona, R.R., J. Pan, A.R. Zota, J.M. Schwartz and M. Friesen et al., 2016. Direct measurement of bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health.
- Mourad, I.M. and Y.A. Khadrawy, 2012. The sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of bisphenol A. Int. J. Life Sci. Pharma Res., 2: L19-L28.
- Wang, T., M. Li, B. Chen, M. Xu and Y. Xu et al., 2012. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab., 97: E223-E227.
- Braun, J.M., A.E. Kalkbrenner, A.M. Calafat, K. Yolton, X. Ye, K.N. Dietrich and B.P. Lanphear, 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics, 128: 873-882.
- Jung, K.K., S.Y. Kim, T.G. Kim, J.H. Kang, S.Y. Kang, J.Y. Cho and S.H. Kim, 2007. Differential regulation of thyroid hormone receptor-mediated function by endocrine disruptors. Arch. Pharm. Res., 30: 616-623.
- Oguazu, C.E., F.C. Ezeonu, C.C. Dike and C.G. Ikimi, 2021. Bisphenol A exposure causes increased oxidation of low density lipoprotein (LDL) and its abduct in rats. Asian J. Res. Biochem., 9: 1-10.
- Lang, I.A., T.S. Galloway, A. Scarlett, W.E. Henley, M. Depledge, R.B. Wallace and D. Melzer, 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. J. Am. Med. Assoc., 300: 1303-1313.
- Akingbemi, B.T., C.M. Sottas, A.I. Koulova, G.R. Klinefelter and M.P. Hardy, 2004. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat leydig cells. Endocrinology, 145: 592-603.
- Rajakumar, C., H. Guan, D. Langlois, M. Cernea and K. Yang, 2015. Bisphenol A disrupts gene expression in human placental trophoblast cells. Reprod. Toxicol., 53: 39-44.
- Li, D., Z. Zhou, D. Qing, Y. He and T. Wu et al., 2009. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod., 25: 519-527.
- Rebai, I., J.O. Fernandes, M. Azzouz, K. Benmohammed, G. Bader, K. Benmbarek and S.C. Cunha, 2021. Urinary bisphenol levels in plastic industry workers. Environ. Res.
- Castro, B., P. Sánchez, J.M. Torres, O. Preda, R.G. del Moral and E. Ortega, 2013. Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS ONE.
- El-Beshbishy, H.A., H.A. Aly and M. El-Shafey, 2013. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol. Ind. Health, 29: 875-887.
- Yang, M., M. Chen, J. Wang, M. Xu and J. Sun et al., 2016. Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology, 157: 2333-2345.
- Chou, W.C., J.L. Chen, C.F. Lin, Y.C. Chen, F.C. Shih and C.Y. Chuang, 2011. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: A birth cohort study in Taiwan. Environ Health.
- Invernizzi, P., 2013. Liver auto-immunology: The paradox of autoimmunity in a tolerogenic organ. J. Autoimmunity, 46: 1-6.
- Rashid, H., F. Ahmad, S. Rahman, R.A. Ansari and K. Bhatia et al., 2009. Iron deficiency augments bisphenol A-induced oxidative stress in rats. Toxicology, 256: 7-12.
- Hasselberg, L., S. Meier and A. Svardal, 2004. Effects of alkylphenols on redox status in first spawning Atlantic cod (Gadus morhua) Aquat. Toxicol., 69: 95-105.
- Khan, S., S. Beigh, B.P. Chaudhari, S. Sharma and S.A.H. Abdi et al., 2016. Mitochondrial dysfunction induced by bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol., 31: 1922-1934.
- Kim, J.H., M.R. Lee and Y.C. Hong, 2016. Modification of the association of bisphenol A with abnormal liver function by polymorphisms of oxidative stress-related genes. Environ. Res., 147: 324-330.
- Veiga-Lopez, A., L.J. Luense, L.K. Christenson and V. Padmanabhan, 2013. Developmental programming: Gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology, 154: 1873-1884.
- Kabuto, H., S. Hasuike, N. Minagawa and T. Shishibori, 2003. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ. Res., 93: 31-35.
- Hassan, Z.K., M.A. Elobeid, P. Virk, S.A. Omer, M. ElAmin and M.H. Daghestani, 2012. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med. Cell. Longevity.
- Oguazu, C.E., F.C. Ezeonu, A.B. Azuka, D.C. Charles and A.N. Onuabuchi et al., 2020. Oxidative stress markers alteration by Bisphenol A exposure predisposes female albino rats to potential risks of oxidative injury. IOSR J. Environ. Sci. Toxicol. Food Technol., 14: 20-27.
- Zorov, D.B., M. Juhaszova and S.J. Sollott, 2006. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA) Bioenerg., 1757: 509-517.
- Kourouma, A., C. Quan, P. Duan, S. Qi, T. Yu, Y. Wang and K. Yang, 2015. Bisphenol A induces apoptosis in liver cells through induction of ROS. Adv. Toxicol.
- Wu, J.H., X.R. Jiang, G.M. Liu, X.Y. Liu, G.L. He and Z.Y. Sun, 2011. Oral exposure to low-dose bisphenol A aggravates testosterone-induced benign hyperplasia prostate in rats. Toxicol. Ind. Health, 27: 810-819.
- Eid, J.I., S.M. Eissa and A.A. El-Ghor, 2015. Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Appl. Zool., 71: 10-19.
- Ezz, H.S.A., Y.A. Khadrawy and I.M. Mourad, 2015. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology, 67: 145-155.
- Abedelhaffez, A.S., E.A.A. El-Aziza, M.A.A. Aziz and A.M. Ahmed, 2017. Lung injury induced by Bisphenol A: A food contaminant, is ameliorated by selenium supplementation. Pathophysiology, 24: 81-89.
- Chitra, K.C., C. Latchoumycandane and P.P. Mathur, 2003. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology, 185: 119-127.
- Yokota, H., H. Iwano, M. Endo, T. Kobayashi, H. Inoue, S.I. Ikushiro and A. Yuasa, 1999. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J., 340: 405-409.
- Yuehui, F., Z. Yiting, Z. Yan, G. Xin and T. Tao, 2013. Effect of vitamin E on reproductive functions and anti-oxidant activity of adolescent male mice exposed to bisphenol A. J. Hyg. Res., 42: 18-22.
- Pigeolet, E., P. Corbisier, A. Houbion, D. Lambert and C. Michiels et al., 1990. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mechanisms Ageing Dev., 51: 283-297.
How to Cite this paper?
APA-7 Style
Oguazu,
C.E., Chukwuemeka,
E.F., Okechukwu,
E.M., Charles,
D.C., Ikechukwu,
U.K. (2023). Monitoring of Low-Dose Effects of Bisphenol a on Superoxide Dismutase and Catalase Level in Female Rats. Asian Journal of Biological Sciences, 16(1), 10-17. https://doi.org/10.3923/ajbs.2023.10.17
ACS Style
Oguazu,
C.E.; Chukwuemeka,
E.F.; Okechukwu,
E.M.; Charles,
D.C.; Ikechukwu,
U.K. Monitoring of Low-Dose Effects of Bisphenol a on Superoxide Dismutase and Catalase Level in Female Rats. Asian J. Biol. Sci 2023, 16, 10-17. https://doi.org/10.3923/ajbs.2023.10.17
AMA Style
Oguazu
CE, Chukwuemeka
EF, Okechukwu
EM, Charles
DC, Ikechukwu
UK. Monitoring of Low-Dose Effects of Bisphenol a on Superoxide Dismutase and Catalase Level in Female Rats. Asian Journal of Biological Sciences. 2023; 16(1): 10-17. https://doi.org/10.3923/ajbs.2023.10.17
Chicago/Turabian Style
Oguazu, Chinenye, E., Ezeonu Francis Chukwuemeka, Enemali Micheal Okechukwu, Dike Chijioke Charles, and Ubaoji Kingsley Ikechukwu.
2023. "Monitoring of Low-Dose Effects of Bisphenol a on Superoxide Dismutase and Catalase Level in Female Rats" Asian Journal of Biological Sciences 16, no. 1: 10-17. https://doi.org/10.3923/ajbs.2023.10.17

This work is licensed under a Creative Commons Attribution 4.0 International License.



