Purification and Determination of Antioxidant Effects of Ethanol Extract Fractions in Phyllanthus Amarus Leaves
| Received 18 Jun, 2022 |
Accepted 26 Jul, 2022 |
Published 01 Nov, 2022 |
Background and Objective: All organisms have an antioxidant defense mechanism that uses enzymes like superoxide dismutase and catalase, as well as substances like ascorbic acid, tocopherol and glutathione, to protect them from free radical damage. Consumption of more antioxidants (phytochemicals) from herbs like Phyllanthus amarus, meals or supplements boosts the body’s antioxidant defense mechanism, protecting it against toxic ROS. The goal of this study was to see if there are any antioxidants in the ethanolic extract fractions of Phyllanthus amarusleaves. Materials and Methods: The leaves of Phyllanthus amaruswere taken from the Federal University Wukari Campus in Taraba State, Nigeria, dried and crushed before being subjected to anti-lipid peroxidation inhibition assay, β-carotene bleaching inhibition assay, total phenolic content determination, total flavonoid content determination and total antioxidant capacity determination further tests. Results: According to the findings of this study, fraction 4b (445.9459%) has the maximum bleaching inhibition power for beta-carotene inhibition testing, whereas, fraction 4a (81.08109%) has the lowest. Fraction 2a (56.9565 mg mL‾1) has the highest phenolic content while fraction 6b (7.608669 mg mL‾1) has the lowest phenolic content. The assay for total flavonoid content showed that fraction 3a (145.71428 mg mL‾1) has the highest flavonoid content while fraction 8a (121.42857 mg mL‾1) has the lowest flavonoid content. The results for anti-lipid peroxidation revealed that fraction 3b (55.732 mg mL‾1) has the highest antioxidant capacity while fraction 6a (2.775 mg mL‾1) has the lowest antioxidant activity. The results of the analysis revealed that Phyllanthus amarusleaves contain significant nutritional components that are good for human health. Conclusion: It is reasonable to conclude that fractions 2a and 2b have the highest antioxidant activities which have good potential for drug development, especially against diseases of oxidative stress origin.
| Copyright © 2022 Ejeh et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The development of novel plant natural products with an economic value has been aided by the research of phytochemicals in numerous industries such as traditional and alternative medicine, pharmaceuticals, nutraceuticals and dietary supplement industries1. The importance of phytochemistry in the medical and pharmaceutical sectors for the development of novel medications has overtaken its importance in other industries due to the constant danger of microbes and environmental threats to human health. Approximately 80% of 122 plant-derived medications, according to Fabricant and Farnsworth2, are connected to their original traditional applications. According to reports, 11% of the 252 medications recognized as basic and necessary by the World Health Organization (WHO) around the turn of the century were only derived from flowering plants1. In Nigeria, rural inhabitants utilize various portions of Phyllanthus amarus for medicinal reasons in the treatment/management of various ailments3.
Phyllanthus is a member of the Euphorbiaceae family, which includes erect and prostrate plants and shrubs with milky acrid juice. Plants of the Phyllanthus genus are either monoecious or homoecious. They have simple, alternating or opposite leaves. Their blooms are diclinous, tiny and grouped in cup-shaped formations. They are greenish and often include glands. The fruit of the Phyllanthus plant is a three-lobed capsule that extends from the cup and has a long stem4. This plant has been shown to contain a variety of medicinally important chemical substances, including alkaloids, flavonoids, lactones, steroids and terpenoids. However, the most prevalent substances discovered so far in this genus include lignans, triterpenes, alkaloids and tannins1.
Reactive Oxygen Species (ROS) such as superoxide radical anion and hydroperoxyl radicals are produced in cells as byproducts of metabolism and are responsible for a variety of degenerative disorders including Cardiovascular Disease (CVD) and diabetes, cirrhosis and malignancies5. They may be created by the mitochondrial respiratory chain, photochemical and enzymatic reactions, exposure to UV light, ionizing radiation or heavy metal ions, among other mechanisms. By simulating corneal cell death, ROS contribute to the development of keratoconus, Fuchs endothelial corneal dystrophy and granular corneal dystrophy type 2 in adults. To sustain physiological functions, such as proliferation, host defense, signal transmission and gene expression, low amounts of ROS generation are essential6. Under normal conditions, there is a biological balance between ROS generation and clearance and eukaryotic cells have a variety of antioxidative defense mechanisms, including enzymes and antioxidants. However, oxidative stress occurs when cellular ROS overproduction surpasses intrinsic antioxidant capacity, which may lead to damage to biomolecules in healthy cells and tissues1. Oxidative stress can be brought on by an excessive amount of ROS production, mitochondrial dysfunction, a compromised antioxidant system or a combination of these causes. The peroxidative/antioxidative cellular imbalance between ROS generation and the capacity of biological systems’ defense mechanisms to remove cellular stress disturbances results in a vicious cycle since oxidative stress reciprocally worsens ROS production6.
All organisms have an antioxidant defense mechanism that uses enzymes like superoxide dismutase and catalase, as well as substances like ascorbic acid, tocopherol and glutathione, to protect them from free radical damage7. However, our bodies’ systems occasionally fail to function properly, resulting in inevitable oxidative damage. As a result, consuming more antioxidants (phytochemicals) through herbs, meals or supplements is advantageous to the body system, as it protects it from these damaging ROS6. Although there have been some publications on the health advantages of Phyllanthus amarus, there is little information on its antioxidant content. The goal of this study was to see if there were any antioxidants in the ethanolic extract fractions of Phyllanthus amarus leaves.
MATERIALS AND METHODS
Study area: The study was carried out in the Central Research Laboratory located in Federal University Wukari, Taraba State, Nigeria between January and April, 2022.
Plant collection: Phyllanthus amarus leaves were collected at Federal University, Wukari Premise, Taraba State, Nigeria. The dried leaves were crushed into a fine powder manually, using mortar and pestle as described by Oduola et al.8 (Fig. 1).
 |
Fig. 1: Phyllanthus amarus plant9 |
Preparation of ethanolic extract: Exactly 415 g of the pulverized sample was soaked in about 1150 mL of 99.8% ethanol after which the post ethanolic residue was soaked in 99.8% chloroform for 48 hrs using the same measurements. The extracts were first sieved using a mesh of which the juice obtained was further filtered using Whatman number 1 filter paper to obtain the filtrate. The ethanolic extract was placed under the fan to evaporate the ethanol before subjecting it to further experiment.
Fractionation of ethanolic extract: Packing of the column and elution was done according to the method of Khan et al.9. To separate the ethanolic extract into its component fractions, column chromatography was used. The column was packed with silica gel and the mobile phase was made up of several solvent mixtures with increasing polarity. The eluted fractions were collected in aliquots of 100 mL in each of the beakers.
Determination of total antioxidant capacity: The experiment was carried out in duplicate according to the procedure of Chang et al.10. About 0.02 g of DPDH was dissolved in 100 mL of methanol then 2 mL of DPPH solution was added into test tubes and 100 μL of each fraction was added. The mixture was shaken vigorously and the absorbance read within 30 sec at 517 nm in a UV-visible spectrophotometer. Methanol was used to zero the reading and the absorbance of the DPPH solution was measured and recorded as blank.
Determination of total flavonoid content: The aluminium chloride colourimetric technique developed by Chavan et al.11 was used to determine the total flavonoid concentration. The calibration curve was derived using a quercetin standard and the total flavonoid concentration was represented as mg mL–1 quercetin equivalent (QE).
Determination of total phenolic content: The Total Phenolic Content (TPC) of the extract was estimated following the procedure of Velioglu et al.12. Garlic acid was used as standard and the data was calculated as garlic equivalence in μg.
β-carotene bleaching inhibition assay: The method of Jayaprakasha et al.13 was used in assaying for β-carotene inhibition. In this assay, antioxidant activity was determined by measuring the inhibition of conjugated diene hydroperoxides arising from linoleic acid oxidation.
The bleaching capacity was calculated in age (%):
Where:
| AS120 | = |
Absorbance of the test sample after incubation for 120 min |
| AS120 | = |
Absorbance of control after incubation for 120 min |
| AC0 | = |
Absorbance of control before incubation for 120 min |
Anti-lipid peroxidation inhibition assay: This assay was used to estimate the lipid peroxidation of the plant extract by following the methods of dos-Reis et al.14. Anti-lipid peroxidation was assessed using the formula:
Where:
| Ai | = |
Absorbance of Fe2+ induced peroxidation |
| As | = |
Absorbance of test sample |
| Ac | = |
Absorbance of control |
RESULTS AND DISCUSSION
Total Antioxidant Capacity (TAC): The results for total antioxidant capacity revealed that F1a has the highest antioxidant capacity, followed by F3b and then F2a with the values of 61 mg Trolox mL–1 of n-hexane fraction of the chloroform extract, 55.732 mg Trolox mL–1 of chloroform fraction of the chloroform extract and 55 mg Trolox mL–1 n-hexane:chloroform (50:50 solvents combination), respectively. On the other hand, F6a (2.775 mg mL–1) has the lowest antioxidant capacity. Fraction 1 had a solvent combination of chloroform (100:00), fraction 3 with a solvent combination of chloroform (100:00) while fraction 2 with a solvent combination of n-hexane:chloroform (50:50). These results were represented in Fig. 2.
 |
Fig. 2: Total antioxidant capacity of Phyllanthus amarus leaves F1a: n-hexane 100:00, F1b: n-hexane 100:00, F2a: n-hexane:chloroform 50:50, F2b: n-hexane:chloroform 50:50, F3a: Chloroform 100:00, F3b: Chloroform 100:00, F4a: Chloroform:ethyl acetate 50:50, F4b: Chloroform:ethyl acetate 50:50, F5a = Ethyl acetate 100:00, F5b = Ethyl acetate 100:00, F6a = Ethyl acetate:ethanol 50:50, F6b: Ethyl acetate:ethanol 50:50, F7a: Ethanol 100:00, F7b: Ethanol 100:00, F8a: Ethanol:methanol 50:50, F8b : Ethanol:methanol 50:50 |
The total antioxidant capacity of the fractions of chloroform extract of Phyllanthus amarus varied greatly from one another. This was in line with the studies of Dos-Reis et al.14. This means it contains a significant number of bioactive substances, including polyphenols (flavonoids), which were recognized for their antioxidant properties15,16. This was in line with certain research showing that medicinal plants used in traditional and alternative medicine are high in antioxidants. The results also revealed that F3b has the highest antioxidant value, indicating that the chloroform solvent combination is more efficient in the extraction of antioxidant phytochemicals from Phyllanthus amarus than the ethyl acetate:ethanol 50:50 solvent combination, which has the lowest extraction ability as evident in F6a and thus the efficiency of chloroform in this extraction could be due to the non-polarity of some of the antioxidant phytochemicals (beta-carotene).
These data suggested that the extraction solvents may have a substantial impact on the variation in phytochemical concentration and partitioning in various solvents from P. amarus leaves. These results were consistent with recent research on Limnophila aromatic Do et al.3 and Phoenix dactylifera17, in which variance was accounted for by differences in solubility of the various chemicals in the sample. Antioxidant protection in the cell is mediated by physiological antioxidant enzymes (GPX, SOD, CAT and GSH). Antioxidants are responsible for the organism’s defensive systems against diseases caused by free radical attacks. As a result, consuming antioxidants produced from plants aids in the prevention of degenerative disorders that can contribute to oxidative stress such as cancer, Parkinson’s, Alzheimer or atherosclerosis18,19.
Total Phenolic Content (TPC): The F2a (56.9565 mg QAE mL–1 chloroform extract), F1b (35.652 mg QAE mL–1 chloroform extract) and F4a (29.1304 mg QAE mL–1 chloroform extract) had the greatest total phenolic content, followed by F1b (35.652 mg QAE mL–1 chloroform extract) and F4a (29.1304 mg QAE mL–1 chloroform extract). The phenolic concentration of F6b (7.60869 mg QAE mL–1 chloroform extract) is the lowest. F1b has a solvent combination of n-hexane (100:00), F2a has a solvent combination of n-hexane:chloroform (50:50), F4a has a solvent combination of chloroform:ethyl acetate (50:50), F6b has a solvent combination of ethyl acetate:ethanol (50:50), F4b chloroform:ethyl acetate (50:50), (100:00). Figure 3 depicted this situation. The phrase phenolic acid refers to phenolic compounds that include only one carboxylic group.
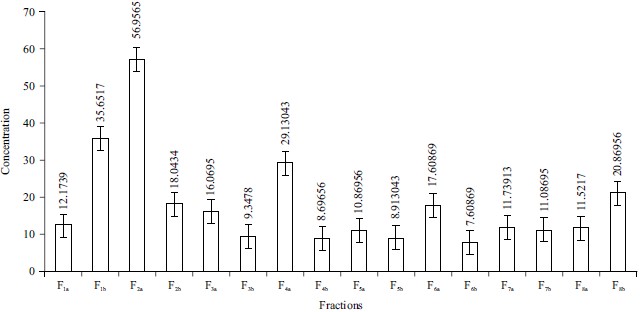 |
Fig. 3: Total phenolic content of Phyllanthus amarus leaves F1a: n-hexane 100:00, F1b: n-hexane 100:00, F2a: n-hexane:chloroform 50:50, F2b: n-hexane:chloroform 50:50, F3a: Chloroform 100:00, F3b: Chloroform 100:00, F4a: Chloroform:ethyl acetate 50:50, F4b: Chloroform:ethyl acetate 50:50, F5a: Ethyl acetate 100:00, F5b: Ethyl acetate 100:00, F6a: Ethyl acetate:ethanol 50:50, F6b: Ethyl acetate:ethanol 50:50, F7a: Ethanol 100:00, F7b: Ethanol 100:00, F8a: Ethanol:methanol 50:50, F8b: Ethanol:methanol 50:50 |
One of the primary groups of plant phenolics is phenol carboxylic acids (a form of phytochemical known as polyphenol). Several soluble polyphenols, including ferulic acid, gallic acid and flavonoids, have been reported to either enhance or inhibit spore germination and hyphal development in saprotrophic fungus. Specific phenolics generated by competitors can impede plant mycorrhizal infection, nutrient absorption and plant development20.
The total phenolic content in the different fractions of the chloroform extract of Phyllanthus amarus using Folin-Ciocalteu’s reagent is expressed in terms of gallic acid equivalent (the standard curve equation):
The values obtained for the concentration of total phenols are expressed as mg of GA mL–1 of extract as shown in Fig. 3. Total values for a total phenolic content range from 9.3478 mg GAE mL–1 n-hexane (100:00) fraction to 56.9565 mg GAE mL–1 ethanol:methanol (50:50) fraction. These results were in agreement with previous studies that nonpolar solvents have low extraction ability for polyphenolic compounds compared to solvent combinations of ethanol and methanol21. The high concentration of phenolic compounds was a result that the genus Phyllanthus amarus is known for its high phenolic content22. The presence of phenol indicates that Phyllanthus amarus can be used in medicines to treat a variety of medical problems. Because phenols are widely recognized for their huge power to battle cancer, the presence of phenols in the plant makes it a possible cancer treatment. Antioxidants are derivatives of phenolics and their derivatives particularly phenolic acids, flavonoids and other phenolics23. Phenolic compounds are known to exhibit direct antioxidant activity by inducing endogenous protective enzymes and a positive regulatory effect on signalling pathways24. Phenolic acids act as antioxidants due to their phenol moiety23.
Total Flavonoid Content (TFC): The results for total flavonoid content show that F5a (147.142857 mg QE mL–1 the chloroform extract) with the solvent combination ethyl acetate 100:00 has the highest flavonoid content followed by F3a (145.71428 mg QE mL–1 chloroform extract) with a solvent combination of chloroform 100:00 and then F2b (145.0 mg QE mL–1 chloroform extract) with the solvent combination of n-hexane:chloroform 50:50. While, F8a (121.42857 mg QE mL–1 chloroform extract) with the solvent combination of ethanol:methanol 50:50 has the lowest flavonoid content followed by F7b (122.14285 mg QE mL–1 chloroform extract) with the solvent combination of ethanol 100:00 and then F8b (124.28571 mg QE mL–1 chloroform extract). These results were represented in Fig. 4.
A significant group of natural products are flavonoids, specifically, they are a group of secondary plant metabolites with a polyphenolic structure that are prevalent in fruits, vegetables and certain drinks. They have a variety of beneficial biochemical and antioxidant properties linked to several disorders, including atherosclerosis, Alzheimer’s Disease (AD) and cancer25. They belong to a class of low-molecular-weight phenolic compounds that are widely distributed in the plant kingdom. They constitute one of the most characteristic classes of compounds in higher plants23. With C-4 keto groups and either the C-3 or C-5 hydroxide group of flavones and flavonoids, aluminium chloride produces acid-stable complexes. This study demonstrated that F5a has the highest flavonoid concentration compared with F5b. This observation is an indication of the possibility that F5a could exhibit antioxidant activity as asserted by the GCMS and FTIR analysis of Phyllanthus amarus methanol extract26, where flavonoid presence was confirmed.
This study showed that the extraction solvents had a significant effect on the extraction of flavonoids. The high flavonoid content in F5a could be attributed to the high extractability potentials of the solvent (ethyl acetate 100%). The greatest quantities of flavonoids were extracted from P. amarus using absolute ethyl acetate. Previous research on S. chinensis fruit pulp, Limnophila aromatica and Macadamia tetraphylla skin waste, which found that extraction solvents greatly impacted flavonoids, confirmed our findings3,25,27.
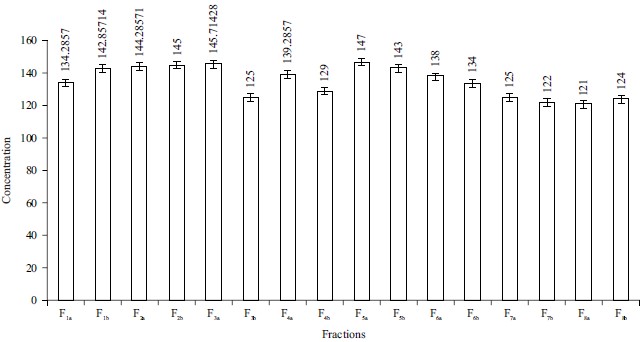 |
Fig. 4: Total flavonoid content of Phyllanthus amarus leaves F1a: n-hexane 100:00, F1b: n-hexane 100:00, F2a: n-hexane:chloroform 50:50, F2b: n-hexane:chloroform 50:50, F3a: Chloroform 100:00, F3b: Chloroform 100:00, F4a: Chloroform:ethyl acetate 50:50, F4b: Chloroform:ethyl acetate 50:50, F5a: Ethyl acetate 100:00, F5b: Ethyl acetate 100:00, F6a: Ethyl acetate:ethanol 50:50, F6b: Ethyl acetate:ethanol 50:50, F7a: Ethanol 100:00, F7b: Ethanol 100:00, F8a: Ethanol:methanol 50:50, F8b: Ethanol:methanol 50:50 |
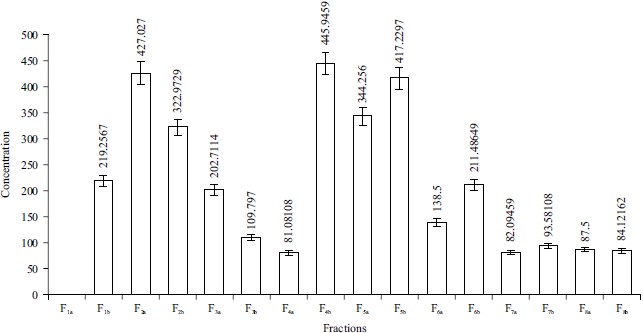 |
Fig. 5: β-Carotene bleaching inhibition assay in different groups F1a: n-hexane 100:00, F1b: n-hexane 100:00, F2a: n-hexane:chloroform 50:50, F2b: n-hexane:chloroform 50:50, F3a: Chloroform 100:00, F3b: Chloroform 100:00, F4a: Chloroform:ethyl acetate 50:50, F4b: Chloroform:ethyl acetate 50:50, F5a: Ethyl acetate 100:00, F5b: Ethyl acetate 100:00, F6a: Ethyl acetate:ethanol 50:50, F6b: Ethyl acetate:ethanol 50:50, F7a: Ethanol 100:00, F7b: Ethanol 100:00, F8a: Ethanol:methanol 50:50, F8b: Ethanol:methanol 50:50 |
The varied polarity of the chemicals, which were specifically more soluble in different solvents, can potentially be used to explain this variance. The difference in the total phenolic content of the fractions further demonstrated that chloroform and n-hexane (50:50) were the most effective solvent for extracting TPC to its highest level from the sample. Previous research had also discovered that various extraction solvents have a substantial impact on TPC extraction yield3,25,27.
Beta-carotene bleaching inhibition assay: The results for beta-carotene bleaching inhibition was shown in Fig. 5. F4b (445.9459%) with the solvent combination chloroform:ethyl acetate 50:50 has the highest percentage bleaching inhibition, followed by F2a (427.027%) with the solvent combination n-hexane:chloroform 50:50 and F5b (417.2297%) with the solvent combination ethyl acetate 100:00. The lowest bleaching inhibition activity is F4b (81.10%) with the solvent combination chloroform:ethyl acetate 50:50, followed by F7a (82.10%) with the solvent combination ethanol 100:00 and F8b (84.12%) with the solvent combination ethanol:methanol 50:50.
Beta-carotene is a terpenoid that is fat-soluble and lipophilic. Superoxide dismutase, for example, is a free radical that is regulated in the biological system by various key enzymes that have antioxidant capabilities. In the absence of antioxidants, beta-carotene undergoes rapid bleaching and decolourization from the formation of hydroperoxides by linoleic acid oxidation. The beta-carotene concentration of Phyllanthus amarus ranged from 445.9-79.05 mg mL–1 indicating that the plant has significant antioxidant potential.
The F1b and F4a had the greatest beta-carotene activity, with concentrations of 445 and 427 mg mL–1, respectively, followed by F2a and F4b with 322 and 344 mg mL–1. The lowest beta-carotene bleaching inhibition activity was found in F8b (79 mg mL–1), followed by F3b (81 mg mL–1). Chloroform removed a higher amount of beta-carotene than any other solvent combination. This was consistent with the findings of previous studies4,25,27, which claimed that various extraction solvents have a substantial impact on phytochemical extraction yield. Both beta-carotene and chloroform are nonpolar solutes. Nonpolar solvents extract a better proportion of nonpolar solutes. This observation indicated that chloroform is the best solvent in the extraction of beta-carotene because of its ability the extraction of nonpolar solutes.
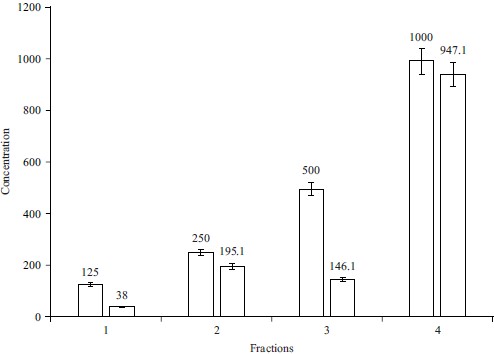
Anti-lipid peroxidation assay: The anti-lipid peroxidation findings with various concentrations of the ethanolic extract was shown in Fig. 6. The effectiveness of the extract to prevent peroxidation grew gradually as the concentration of the extract rose. The skin is chronically exposed to both endogenous and environmental pro-oxidant agents as a result of the interface function between the body and the environment, resulting in the detrimental formation of reactive oxygen species (ROS). There was substantial evidence that oxidative stress plays a role in the cellular destruction of DNA, cell membrane lipids and proteins27. The extract’s percentage of lipid inhibition activity at 1000 g mL–1 was found to have the highest anti-lipid peroxidation activity, while the concentration of 125 g mL–1 had the lowest. This was generally in agreement with other research studies that have investigated the effect of plant extract on meat22,27.
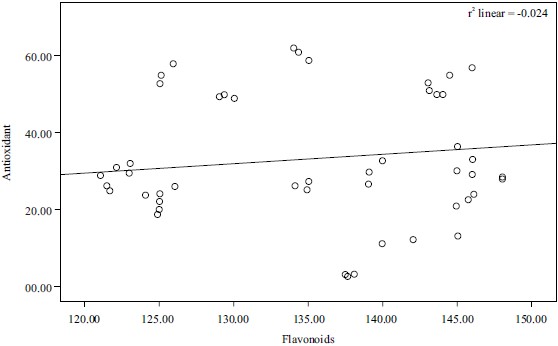 |
Fig. 8: Correlation between antioxidants and flavonoids (r2 = -0.024) |
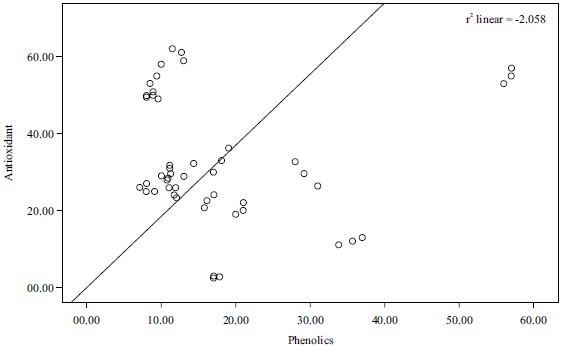
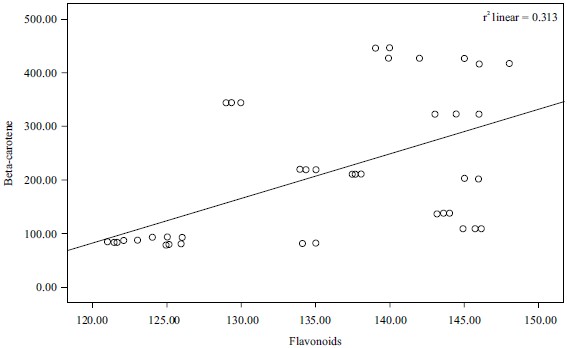
Correlation of results: Pearson’s correlation coefficient (r) was obtained from bivariate correlation analysis and used to describe the correlation between the antioxidant activities and the content of antioxidant components (TAC, TFC and TPC beta-carotene). The correlation between total antioxidants capacity and total phenolic contents and that of antioxidant and flavonoid was found to be weak negative (r2 = -2.058, r2 = -0.024, respectively), while the correlation between phenolics and beta-carotene and that of the total antioxidants and total flavonoids was found to be weak positive (r2 = 0.245 and r2 = 0.313, respectively). These results were represented in Fig. 7-10.
The correlation coefficient between total phenol content and the β-carotene bleaching test of Phyllanthus amarus was significant (r= 0.313). This was in line with the studies28 which further added that the presence of antioxidants will minimize the oxidation of β-carotene. This indicated that the flavonoid contents of Phyllanthus amarus were responsible for the antioxidant activity of the extract.
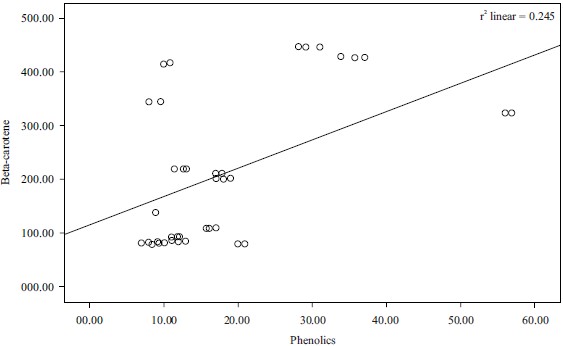 |
Fig. 10: Correlation between beta-carotene and phenolics (r2 = 0.245) |
CONCLUSION
The results of the analysis revealed that Phyllanthus amarus leaves contain significant nutritional components that are good for human health such as flavonoids which is a subclass of the phytochemical, polyphenols. The correlation that exists between beta-carotene bleaching inhibition assay and the total flavonoids (r2 = 0.313) is an indication that the flavonoid content of Phyllanthus amarus extract earlier demonstrated using DPPH is the consequence of the inherent flavonoid content of the plant. It is reasonable to conclude that F3b with the solvent combination (chloroform 100:00) and F2a with the solvent combination (n-hexane:chloroform 50:50) with the highest antioxidant activity have good potential for drug development, especially against the disease of oxidative stress origin.
SIGNIFICANCE STATEMENT
This study discovered the antioxidant effects of Phyllanthus amarus leaves that can be beneficial to human health, protecting cells against free radicals, which may play a role in heart disease and cancer among others. This study will help the researchers to uncover the critical areas of phytomedicine in Phyllanthus amarus leaves that many researchers were not able to explore. Thus a new theory on drug design may be arrived at.
ACKNOWLEDGMENT
We would like to thank Dr. Yakubu Ojochenemi Ejeh for initiating the concept of this research work and spearheading it.
REFERENCES
- Yakubu, O.E., O.F.C. Nwodo, P.E. Joshua, M.N. Ugwu, A.D. Odu and F. Okwo, 2014. Fractionation and determination of total antioxidant capacity, total phenolic and total flavonoids contents of aqueous, ethanol and n-hexane extracts of Vitex doniana leaves. Afr. J. Biotechnol., 13: 693-698.
- Fabricant, D.S. and N.R. Farnsworth, 2001. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect., 109: 69-75.
- Do, Q.D., A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji and Y.H. Ju, 2014. Effect of extraction solvent on total phenol content, total flavonoid content and antioxidant activity of Limnophila aromatica. J. Food Drug Anal., 22: 296-302.
- Sulaiman, C.T., K.V. Thushar, S. George and I. Balachandran, 2014. Phenolic characterisation of selected Salacia species using LC-ESI-MS/MS analysis. Nat. Prod. Res., 28: 1021-1024.
- Halliwell, B., 1996. Antioxidants in human health and disease. Annu. Rev. Nutr., 16: 33-50.
- Sen, T. and S.K. Samanta, 2014. Medicinal Plants, Human Health and Biodiversity: A Broad Review. In: Biotechnological Applications of Biodiversity, Mukherjee, J. (Ed.), Springer, Berlin, Heidelberg, ISBN: 978-3-662-45096-3, pp: 59-110.
- Mau, J.L., H.C. Lin and S.F. Song, 2002. Antioxidant properties of several specialty mushrooms. Food. Res. Int., 35: 519-526.
- Oduola, T., A.A. Muhammad, F. Aiyelabegan, M. Tajudeen and S.O. Okalawon, 2018. Hepatotoxic assessment of Phyllanthus amarus leaf extract in Wistar rats. Eur. J. Med. Plants.
- Khan, R.A., M.R. Khan, S. Sahreen and M. Ahmed, 2012. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J.
- Chang, C.C., M.H. Yang, H.M. Wen and J.C. Chern, 2002. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal.
- Chavan, J.J., U.B. Jagtap, N.B. Gaikwad, G.B. Dixit and V.A. Bapat, 2013. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J. Plant Biochem. Biotechnol., 22: 409-413.
- Velioglu, Y.S., G. Mazza, L. Gao and B.D. Oomah, 1998. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem., 46: 4113-4117.
- Jayaprakasha, G.K., R.P. Singh and K.K. Sakariah, 2001. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem., 73: 285-290.
- dos Reisa, L.C.R., V.R. de Oliveira, M.E.K. Hagen, A. Jablonski, S.H. Flôres and A. de Oliveira Rios, 2015. Carotenoids, flavonoids, chlorophylls, phenolic compounds and antioxidant activity in fresh and cooked broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. Alphina F1). LWT-Food Sci. Technol., 63: 177-183.
- Liu, R.H., 2003. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr., 78: 517S-520S.
- Valko, M., D. Leibfritz, J. Moncol, M.T.D. Cronin, M. Mazur and J. Telser, 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol., 39: 44-84.
- Yakubu, O.E., O.F.C. Nwodo, C. Shaibu, S.V. Tatah, M.A. Abah and S. Gabriel, 2019. In vitro determination of antioxidant activities of the fractions obtained from Adansonia digitata L. (baobab) stem bark ethanolic extract using different parameters. Curr. Trend Biomed. Eng. Biosci.
- Pisoschi, A.M. and G.P. Negulescu, 2012. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem.
- Galati, G. and P.J. O'Brien, 2004. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radical Biol. Med., 37: 287-303.
- Veitch, N.C., 2010. Flavonoid Chemistry of the Leguminosae. In: Recent Advances in Polyphenol Research, Volume 2, Santos-Buelga, C., M.T. Escribano-Bailon and V. Lattanzio (Eds.), Wiley-Blackwell, Hoboken, New Jersey, ISBN: 9781405193993, pp: 23-58.
- Baba, S.A. and S.A. Malik, 2014. Evaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurroo royle. Saudi J. Biol. Sci., 21: 493-498.
- Kumar, N. and N. Goel, 2019. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep.
- Stevenson, D.E. and R.D. Hurst, 2007. Polyphenolic phytochemicals-just antioxidants or much more? Cell. Mol. Life Sci., 64: 2900-2916.
- Panche, A.N., A.D. Diwan and S.R. Chandra, 2016. Flavonoids: An overview. J. Nutr. Sci.
- Dailey, A. and Q.V. Vuong, 2015. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric.
- Ebrahimzadeh, M.A., S.M. Nabavi, S.F. Nabavi, F. Bahramian and A.R. Bekhradnia, 2009. Antioxidant and free radical scavenging activity of H. officinalis, L. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci., 23: 29-34.
- Hlila, M.B., A. Omri, H.B. Jannet, A. Lamari, M. Aouni and B. Selmi, 2013. Phenolic composition, antioxidant and anti-acetylcholinesterase activities of the Tunisian Scabiosa arenaria. Pharm. Biol., 51: 525-532.
- Matthews, H.B., G.W. Lucier and K.D. Fisher, 1999. Medicinal herbs in the United States: Research needs. Environ. Health Perspect., 107: 773-778.
How to Cite this paper?
APA-7 Style
Ejeh,
Y.O., Olawale,
O., Umaru,
I.J., Abu,
M.S., Adondua,
M.A., Terhemba,
E. (2022). Purification and Determination of Antioxidant Effects of Ethanol Extract Fractions in Phyllanthus Amarus Leaves. Asian Journal of Biological Sciences, 15(5-6), 259-270. https://doi.org/10.3923/ajbs.2022.259.270
ACS Style
Ejeh,
Y.O.; Olawale,
O.; Umaru,
I.J.; Abu,
M.S.; Adondua,
M.A.; Terhemba,
E. Purification and Determination of Antioxidant Effects of Ethanol Extract Fractions in Phyllanthus Amarus Leaves. Asian J. Biol. Sci 2022, 15, 259-270. https://doi.org/10.3923/ajbs.2022.259.270
AMA Style
Ejeh
YO, Olawale
O, Umaru
IJ, Abu
MS, Adondua
MA, Terhemba
E. Purification and Determination of Antioxidant Effects of Ethanol Extract Fractions in Phyllanthus Amarus Leaves. Asian Journal of Biological Sciences. 2022; 15(5-6): 259-270. https://doi.org/10.3923/ajbs.2022.259.270
Chicago/Turabian Style
Ejeh, Yakubu, Ojochenemi, Otitoju Olawale, Isaac John Umaru, Michael Sunday Abu, Moses Abah Adondua, and Ezekiel Terhemba.
2022. "Purification and Determination of Antioxidant Effects of Ethanol Extract Fractions in Phyllanthus Amarus Leaves" Asian Journal of Biological Sciences 15, no. 5-6: 259-270. https://doi.org/10.3923/ajbs.2022.259.270

This work is licensed under a Creative Commons Attribution 4.0 International License.