Effects of Synergistic Consumption of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on Hematological Indices in Male Rat Model
| Received 17 Nov, 2023 |
Accepted 15 Jan, 2024 |
Published 31 Mar, 2024 |
Background and Objective: The 80% of the population worldwide depend heavily on the use of herbs and supplements singly or as combinations for therapeutic purposes (ameliorative or prophylactically), enhancement or as supplements. This study assessed the effects of a synergistic mixture of Cyperus escunlentus, Phoenix dactylifera and Cocos nucifera (STDC) on selected hematological indices in a male rat model. Materials and Methods: Acute toxicity LD50 of STDC was done and thereafter 15 healthy male Wistar rats weighing between 230-250 g procured from the Pharmacology and Therapeutics Department of the Rivers State University Port Harcourt were allotted into 3 groups containing 5 rats in each group: Control, STDC200 mg/kg and STDC 400 mg/kg groups. The test rats were administered doses of STDC with feed and water as required for 21 days after which blood samples were taken for hematological analysis using standard laboratory procedures. Descriptive statistics were computed and expressed as Mean±SD. One-way Analysis of Variance (ANOVA) and Tukey’s test were performed. The p<0.05 was considered statistically significant. Results: The acute toxicity LD50 as >2404.2 mg/kg while WBC and differential counts (neutrophils, lymphocytes, basophils and eosinophils) were non-significantly (p<0.05) elevated, except monocyte having a non-significantly (p<0.05) reduced value. The RBC, PCV and Hb also manifested a statistically non-significant (p<0.05) increased plasma concentrations as well as a non-significant (p<0.05) decrease in platelet count. Conclusion: Thus, STDC could be useful in improving immunity as well as reticulocyte synthesis and functions.
| Copyright © 2024 Lemii et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The 80% of the population worldwide has depended heavily on the use of herbs and supplements in the past 3 decades as a solution to their primary healthcare1. Various reasons according to Bandaranayake2 were searched and seen to be factors responsible for the tremendous patronage of herbal medications and supplements which include various claims of its efficacy, preference for natural products and high cost of orthodox drugs among others. The combination of these substances is used in various medical and non-medical conditions, for instance, its combination is use as an enhancer of male sexual performance.
Tiger nut (Cyperus escunlentus) is grown and used worldwide because of its high yields and its prospect for multiple utilization. Tiger nuts grow as a tiny tuber and could be eaten fresh, dried, roasted or made in aqueous milky form as sweetmeat1. The nutrient constituent of tiger nuts revealed the following contents 22.14-44.92% lipids, 3.28-8.45% proteins, 23.21-48.12% starch, 8.26-15.47% fibers and 1.60-2.60% ashes, bioactive substances such as organic acids, alkaloids and phenols3,4. Tiger nut is rich in edible oil, like that of olive oil, contains starch, a relatively small amount of protein and is found to be suitable for diabetics and patients with digestive abnormalities and useful in the prevention of cardiac diseases5-8. The fiber content of tiger nuts is found useful in the prevention of cancer of the colon, obesity and gastrointestinal problems5. Tiger nut is found to contain flavonoids and thus has antioxidant properties9.
Date palm (Phoenix dactylifera): A perennial and flowering plant species belongs to the Arecaceae palm family. Date palm is cultivated by and serves as a staple food and remedy for various conditions for the people in the tropics, subtropics, Europe and most places in the world10-13. Some studies have demonstrated that P. dactylifera has antioxidant and analgesic potentials secondary to its flavonoids and alkaloid constituents, respectively14,15 also found its antibacterial potential using the methanol and acetone extract of the leaves. Previous study Gangwar et al.16 on laboratory investigations on rats, showed the antiulcer properties of P. dactylifera. Ishurd and Kennedy17, who did investigation on isolated purified form of glucagon found it to possess a potent anticancer effect while its antidiabetic activities were demonstrated on alloxan-induced diabetic rats18. Phoenix dactylifera had shown various properties in several animal studies to possess nephroprotective activity19,20, anti-inflammatory activity21, sedative22, induction of labor23 and treatment of Alzheimer’s diseases24.
Coconut (Cocos nucifera) also belongs to the palm family Arecaceae being the only durable of the specie in the Cocos genus25. Coconuts have an international distribution with respect to dispersal, cultivation and consumption26. The edible white, fleshy part of the seed (the endosperm) depending on the stage of maturation is referred to as the "coconut meat", "coconut flesh", or "coconut kernel27. A 100 g fresh coconut serve contains 1,480 kj of energy relatively high in total fat (33 g), especially saturated fat (89% of total fat), along with a moderate quantity of carbohydrates (15 g) and protein (3 g). Micronutrients embedded within coconut endosperm in substantial amount include the dietary minerals, manganese, copper, iron, phosphorus, selenium and zinc28. Reports of its antioxidant, anti-inflammatory and cytoprotective effect of coconut extract29 and partial but constant evidence of usage of coconut oil as the topical preventive therapy/treatment of atopic dermatitis and dental caries30 have been made. However, investigations have equally emerged about the role of coconut oil in potentiating the risk of increased plasma low-density lipoprotein cholesterol, consequently increasing the risk of cardiovascular diseases31.
The previous studies presented above have revealed widespread use of tiger nuts, palm dates and coconuts as edible food singly and in combinations/increased amounts as therapy for various diseases or body disorders. Despite these above assertions studies on this novel combinations and inconsistent/paucity reports of studies on the effects of these substances on hematological indices still prevail and thus the reason for the evaluation of the likely beneficial or untoward effects on the hematological parameters in male rat model.
MATERIALS AND METHODS
Study duration and location: This study was carried out at the Department of Pharmacology and Therapeutics animal house, Faculty of Basic Clinical Sciences and Department of Biochemistry Research laboratory, Faculty of Science, Rivers State University from October, 2022 to January, 2023.
Acute toxicity studies (LD50) of STDC: The acute toxicity of a synergistic mixture of Cyperus escunlentus, Phoenix dactylifera and Cocos nucifera (STDC) using Lorke32 Method as reported by Adefisayo et al.33 was used. The Rats were divided into 2 phases of 9 and 8 rats, respectively. In the phase 1 study, 9 rats were divided into 3 groups of 3 rats each and they were administered with STDC by oral gavage at the doses of 10, 100 and 1000 mg/kg. In the phase 2 study, 8 rats were divided into 4 groups of 2 rats each and they were administered with STDC by gavage at the doses of 850, 1700, 3400 and 6800 mg/kg. The general behavior of the animals was observed for the first 1 hour, 4 hrs and hourly in a continuous, intermittent and hourly pattern respectively for the next 24 hrs after administration. The LD50 was determined using the formula:
Where, a is least dose that killed any rat and b is highest dose that did not kill any rat.
Measurement of body weight change: Weekly weight measurement of animals in all groups using a digital weighing balance was done to ascertain either weight gain or weight loss.
Experimental design: The 15 healthy male Albino Wistar rats weighing between 250-300 g were procured from the animal house of the Department of Pharmacology and Therapeutics, Rivers State University, Port Harcourt, Nigeria. The animals were housed in clean plastic cages, placed in well-ventilated house conditions with ambient temperatures of 12 hrs light and dark cycles with unrestricted access to rat pellets and tap water ad libitum. The animals were acclimatized for 7 days and handled according to National Institute of Health (NIH) guidelines for the care and use of experimental animals National Research Council (NRC), 201134.
Plant material and preparation of extract: Fresh seeds of tiger nuts, dates and coconut were purchased from Mile 1 Market in Port Harcourt. Tiger nuts (400 g) were cleaned to remove impurities, washed and put in a bowl. The dates (120 g) were cut into two to remove the seeds, then washed and put in a bowl. The coconut (200 g) was removed from the shell, diced and washed. All were pulverized with a manual blender, adding 150 mL of water until a uniform mixture was obtained. The mixture was filtered using a sieve and the resulting filtrate was put into a bottle until use. This synergistic mixture (STDC) was prepared daily for the 21 days of administration35.
Experimental protocol: The acclimatized animals were allotted into Group 1 (control) and were administered with normal rat feed and distilled water, Group 2 STDC were administered 200 mg/kg of the synergistic mixture (tiger nut, dates and coconut) and Group 3 STDC were administered 400 mg/kg of the synergistic mixture (tiger nut, dates and coconut):
• |
Group 1(control): Feed+water ad libitum |
|
• |
Group 2 STDC: 200 mg/kg of STDC |
|
• |
Group 3 STDC: 400 mg/kg of STDC |
The administration in both group 2 and 3 was done orally using an oral gavage tube. The administration lasted for 21 days and animals were sacrificed35.
Collection of samples (blood): Sacrifice was done in three batches, the first was carried out after 7 days, the second after 14 days and the third after 21 days. The animals were sacrificed, followed by decapitation and dissection. Blood samples were collected through cardiac puncture and transferred into Ethylenediaminetetraacetic Acid (EDTA) sample bottles for hematological analysis. Blood for platelets count was collected in sodium citrate bottle and analyzed uncentrifuged35.
Haematological analysis: Hematological analysis was carried out as reported in method used by Odinga et al.35. Parameters that were analyzed for hematological status include White Blood Cell (WBC), Hemoglobin (Hb), Red Blood Cell (RBC), Packed Cell Volume (PCV), White Blood Cell Count (WBC) and differentials (neutrophils, eosinophils, monocytes and lymphocytes) and platelets using an automated hematological machine; hematology analyzer (Abacus Junior Vet 5, Austria).
Statistical analysis: The descriptive results were expressed as Mean±Standard deviation. Data were analyzed using One-way Analysis of Variance (ANOVA) followed by post hoc test using Tukey’s test and p-value less than 0.05 was considered statistically significant. The statistical analysis was performed with the aid of IBM SPSS, Version 25.
Ethical approval: All animals were treated in accordance with the National Institute of Health guide for the care and use of laboratory animals.
RESULTS
Acute oral toxicity test (LD50) of STDC: Orally administrated STDC at concentrations between 10-1700 mg/kg showed no toxicity in the experimental rats. Symptoms of diarrhea, fatigue and 50% mortality were detected in the rats administered 3400 and 6800 mg/kg of STDC. The other 50% were administered 100 mg/kg of lime fruit juice, which resuscitated them. Variation in weight was a prominent feature across the experimental rats in the two phases of the experiment. The oral LD50 of STDC was determined to be >2404.2 mg/kg b.wt., in adult male albino rats as shown in Table 1.
Table 2 outlines the weight difference of the final from the initial of the experimental rats. For the control, a 30.9% weight increase was observed, 8.39% weight increase for the 200 mg/kg STDC extract-administered rats and a 2.86% weight increase for the 400 mg/kg STDC extract-administered rats.
| Table 1: | Acute oral toxicity test (LD50) of STDC | |||
| Number of rats | Dose (mg/kg) | Sex | Mortality | |
| Phase 1 | ||||
| 03-Mar | 10 | Male | 0/3 | |
| 03-Mar | 100 | Male | 0/3 | |
| 03-Mar | 1000 | Male | 0/3 | |
| Phase 2 | ||||
| 02-Feb | 850 | Male | 0/2 | |
| 02-Feb | 1700 | Male | 0/2 | |
| 02-Feb | 3400 | Male | ½ | |
| 02-Feb | 6800 | Male | ½ | |
| LD50 : Lethal dose, | ||||
| LD50 : Lethal dose, LD50 = |
||||
| Table 2: | Percentage body weight difference in experimental rats exposed to synergistic mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera | |||
| Groups | Initial body weight (g) |
Final body weight (g) |
Body weight difference (%) |
| Group 1 (control) | 69.80±7.50 |
121.00±30.83 |
30.95 |
| Group 2 (200 mg/kg STDC) | 88.20±5.12 |
121.20±29.20 |
8.39 |
| Group 3 (400 mg/kg STDC) | 111.80±6.72 |
123.00±11.68 |
2.86 |
| Values are expressed as Mean±Standard deviation | |||
|
|
Table 3 shows the effect of the synergistic extract (STDC) on the total WBC and the differential count in male rat model.
Total WBCs and differentials (neutrophils, eosinophils and lymphocytes) were observed to have a non-significant (p<0.05) increase with increasing doses in rats administered with STDC when compared with the control group. However, monocyte differential expressed a non-significant (p<0.05) STDC dose-dependent decrease in comparison with the control group.
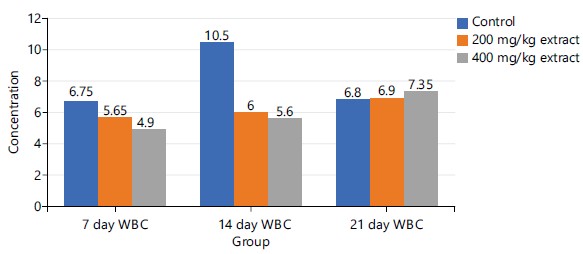
Figure 1 shows the effect of the synergistic extract (STDC) on the total WBC in male rat models at days 7, 14 and 21 days. There is a dose-dependent mild-moderate decrease in the concentration of total WBC on days 7 and 14, however, this trend reversed (dose-dependent increase in WBC concentration) on day 21 in comparison with the control groups.
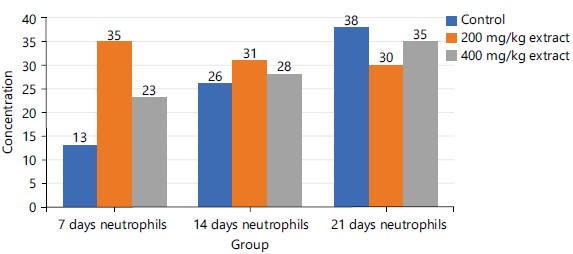
Figure 2 shows that from day 7 to day 14, there was a progressive rise in the concentration of neutrophils in descending order as follows: 200 mg/kg>400 mg/kg extract>control group. However, there is a reversal of this trend at the 21 days of the study as follows: Control>400 mg/kg extract>200 mg/kg extract.
Figure 3 indicated a non-progressive increase in eosinophil concentration in the early phase of treatment (day 7th) but a progressive increase in concentration especially with the 400 mg/kg extract at late phase of treatment 21 days.

|
|
| Table 3: | Effect of ingestion of synergistic extract (STDC) on white blood cells and differential counts in male rat model | |||
| Groups | WBC (cm3) |
Neutrophils (%) |
Eosinophils (%) |
Monocytes (%) |
Lymphocytes (%) |
| Group 1 (Control) | 6.16±1.39a |
23.20±10.64a |
3.00±1.58a |
7.80±2.68a |
68.60±12.72a |
| Group 2 (200 mg/kg STDC) | 6.22±1.07a |
20.00±15.28a |
3.40±1.51a |
5.40±2.30a |
60.60±6.580a |
| Group 3 (400 mg/kg STDC) | 8.20±3.0a |
27.40±5.55a |
4.20±1.79a |
4.80±2.28a |
71.80±18.75a |
| Values with the same superscript alphabets on the same column are not significant at p<0.05, All values were expressed as Mean±Standard Deviation and WBC: White Blood Cells | |||||
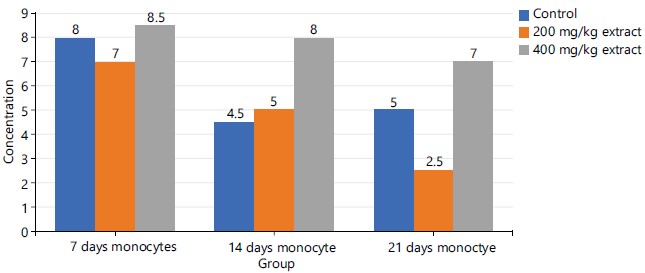
The group 3 (400 mg/kg of STDC) in Fig. 4 maintained a peak but time-dependent decrease in monocyte concentration from day 7 to day 21 of the experimental period while group 2 (200 mg/kg of STDC) showed a progressive decrease in concentration of monocytes for days 7 and day 21 when compared to the control group.
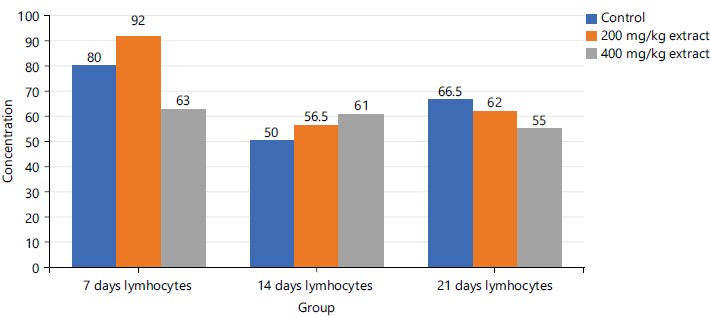
The lymphocyte concentration shows no relative pattern across the study groups at day 7. A progressive increase in concentration with an increase in the dose of STDC is noted at day 14 as follows: 400>200 mg/kg>control group with reversal of this trend at day 21 as indicated in Fig. 5.
Table 4 shows the effect of the synergistic extract (STDC) on the RBCs, PCV, Hb and platelets in male rat models.
|
|
| Table 4: | Effect of synergistic ingestion of synergistic extract (STDC) on red blood cells, platelets and hemoglobin in male Wistar rats | |||
| Groups | RBC (x106/μL) |
Platelet (/μL) |
PCV (%) |
HB (g/dL) |
| Group 1 (Control) | 4.44±0.48a |
349.00±88.29a |
28.00±4.00a |
9.48±1.06a |
| Group 2 (200 mg/kg STDC) | 4.52±0.74a |
225.40±69.23a |
28.80±6.10a |
9.74±1.52a |
| Group 3 (400 mg/kg STDC) | 4.50±1.03a |
344.60±103.17a |
29.00±4.72a |
9.66±1.98a |
| Values are expressed as Mean±Standard Deviation, Values with the same superscript are not significant at p<0.05 levels, RBC: Red blood cell, PCV: Packed Cell Volume and Hb: Hemoglobin | ||||
The RBCs, PCV and Hb were observed to demonstrate a nonsignificant (p<0.05) rise in concentration in the investigated rats administered with doses of STDC when compared to the control group. Platelet however was observed to display a non-significant (p<0.05) reduction in concentration than the control group across the groups.
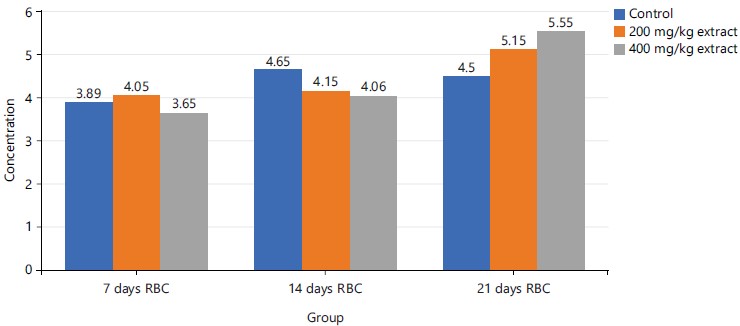
Figure 6 demonstrated a dose-dependent rise in RBC concentration at the late stage (21st day) of the experiment while on days 7 and 14, no pattern of change with respect to doses of STDC was demonstrated across the groups when compared to the control group.
|
|
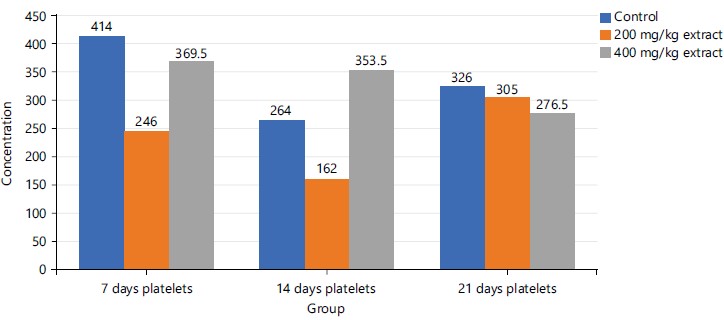
The group 3 (400 mg/kg STDC extract) shows a stepwise time-dependent decrease in platelet concentration on the 21st day when compared to the rest period under study which had fluctuating values in their platelet concentrations in both control and 200 mg/kg STDC extract as shown in Fig. 7.
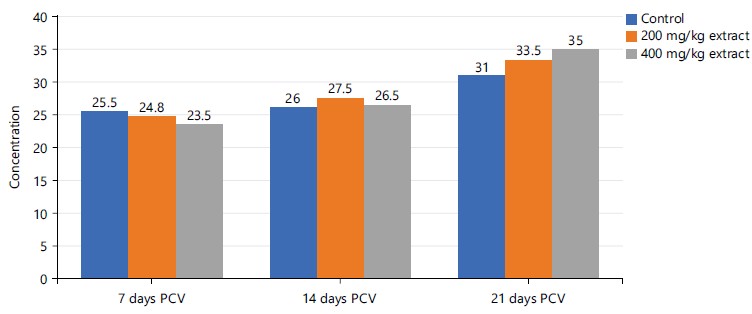
Figure 8 revealed a relative increase in PCV in descending order as 400>200 mg/kg >control groups of STDC extract on the 21st day of the experiment. The PCV values for the 200 mg/kg of STDC extract and the control group expressed no notable difference between the 7 and the 14th day.
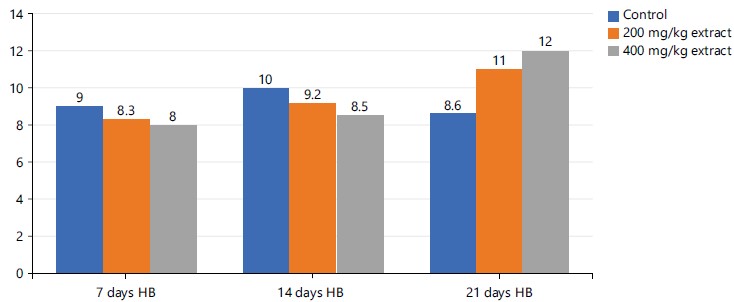
Figure 9 above shows a mild progressive decrease in hemoglobin with increasing dose in descending pattern as follows: Control group >200 mg/kg extract >400 mg/kg STDC extract from days 7 and 14th. There is however reversal of this trend at the late stage (21st day) of the experiment in descending order as: 400 mg/kg extract >200 mg/kg extract >control group.
DISCUSSION
As indicated in Table 1 above, orally administrated STDC at concentrations between 10-1700 mg/kg showed no toxicity in the experimental rats. Symptoms of diarrhea and fatigue and 50% mortality were detected in the rats administered 3400 and 6800 mg/kg of STDC. Lethal dose was seen to be >2404.2 mg/kg from the toxicity test.
|
Table 2 shows that STDC administration led to a decrease in the body weight of the laboratory animals in the following decreasing order: Control group >200 mg/kg STDC >400 mg/kg STDC. The weight difference with reference to the control group could be due to less stress associated with normal feeds and water than with the STDC feeds. Wallis and Hetherington36 reported that stress can also lead to decreased caloric intake in some subjects. Also, the oral gavage method of administration of extract to experimental rats as reported by Murphy et al.37 could incident stress on experimental animals as well as induction of inflammatory conditions or irritations on the upper part of the gut. Thus, a differential decrease in test animals' body weight administered with STDC could be explained by stress possibly imposed on the laboratory animals while feeding the extract rather than the normal feed and water ad libitum.
Table 3 highlighted that synergistic extract (STDC) administered to experimental rats showed a non-significant (p>0.05) increase in WBC concentration (maximal with 400 mg/kg STDC extract) with increasing doses of STDC extract in administered rats when compared with the control group. These findings indicated that a higher dose of the STDC extract could augment WBC in the body. White blood cell and differentials (neutrophils, lymphocytes, monocytes, eosinophils, basophils) parameters are one of the most requested investigations and thus provide useful guides to clinical diagnosis for patients and changes in this index have enormous prognostic value for human health state 38.
Perveen et al.15 found that the methanol and acetone leaf extract of date palm has antibacterial potential. This finding asserted the publications of previous studies39-42 that reported a non-significant increase in total white blood cell count including differential counts (neutrophils, lymphocytes, basophils and eosinophils) in the test group when compared to the control group. The finding of this study also gave credence to the previous studies by researchers43-45, who discovered a dose dependent significant increase of white blood cell concentration and differential counts (neutrophils, lymphocytes, basophils and eosinophils) in their investigated laboratory animals compared to a control group using single or combination of extracts used in this study. Tigner et al.46 noted that WBC circulates in the blood and exerts inflammatory and cellular responses to injury or offending microbes. These findings by implication reveal the possible ability of the STDC constituents in enhancing immune response and defending the body against xenobiotics. The dose-dependent decrease in monocytes as observed in Table 3 is possibly attributable to the presence of coconut extract in the STDC as previously noted by da Silva Lima and Block29 that consumption of coconut oil increased low-density lipoprotein cholesterol (a state of hypercholesteremia) thus as also experimentally detected by Tall and Yvan-Charvet47 that Hypercholesterolemia distorts monocyte/macrophage function, activation and differentiation.
Figures 1-3 and 5 above show some fluctuations in the pattern of spread along the course of the experimental period under consideration with the respective indices, however, their respective cumulative value depicts notable dose-dependent mild-moderate rise in the concentration of WBC, neutrophils, eosinophils and lymphocytes when compared to the control. This finding possibly showed that the process of increase in these indices is time dependent with reference to the STDC extract. Figure 4 above however indicated that group 3 (400 mg/kg of STDC) maintained a peak but time dependent decrease in monocyte concentration from day 7 to day 21 of the experimental period while group 2 (200 mg/kg of STDC) showed a progressive decrease in concentration of monocytes from day 7th to day 21st when compared to the control group. This finding also buttresses the ability of the STDC extract to improve the monocyte count and thus boosting the immune system but tends to progressively decrease (monocytes) at higher STDC extract dosage.
In Table 4, RBCs, PCV and Hb were observed to demonstrate a dose-dependent nonsignificant (p>0.05) rise in concentration in the investigated animals administered with doses of STDC when compared to the control group in a decreasing order as STDC 400 mg/kg>STDC 200 mg/kg>control group. Platelet however was observed to display a non-significant (p>0.05) reduction in concentration than the control group.
The position of this study was consistent with previous studies done on these substances as reported by previous studies40-42 that also demonstrated a non-significant increase in the RBCs, PCV, Hb and Hb indices (MCHC, MCV, MCH) in test group when compared to the controls. The above findings on the effect of STDC extract on hemoglobin parameters also reveal the positive modulatory actions as shown in the studies by researchers39,43,44, who observed not only an increase but statistical significance in the test values when compared with control groups.
Red blood cells produce hydrogen sulfide, a molecule that acts as a vasodilator as asserted by Benavides et al.48 or may boost the immune defense mechanism49. Hemoglobin releases free radicals which lyse and kill offending bacterial membranes49.
This study also revealed a non-significant decrease in platelet with increasing dose of STDC in course of the experiment in a decreasing order as STDC 200 mg/kg
Figures 6, and 8-9 above showed some oscillations in the concentration of RBS, PCV and Hb respectively from day 7 to day 21, however, a cumulative dose and time-dependent mild-moderate rise in the concentration of these indices were noted when compared to the control. This finding possibly showed that the process of increase in these indices is time/dose-dependent with reference to the STDC extract. Figure 7 demonstrated that group 3 (400 mg/kg STDC extract) shows a stepwise time/dose-dependent decrease in platelet concentration when compared to other group 2 and control which had fluctuating values in their platelet concentrations. This finding is also indicative that the STDC extract has the potential to cause thrombocytopenia with increasing dose and time.
CONCLUSION
The discoveries of this study have demonstrated that aqueous mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera (STDC) could serve to enhance red blood cell and hemoglobin indices synthesis, possess bactericidal activities, partake in blood homeostasis and boost or modulate the body immune cells.
SIGNIFICANCE STATEMENT
There is a global quest on ways to improve the general functionality of the body system. A stable hematological status of the human body has been associated with the general wellbeing of human and their health. Plants have been reported to be beneficial therapeutically, hence the need to investigate various natural ways to boost the hematological status. This study evaluated and opined that the intake of the synergistic mixture of Phoenix dactylifera, Cyperus escunlentus and Cocos nucifera (STDC) extract increased the plasma concentrations of RBC, PCV and hemoglobin, thus, STDC could be useful in improving immunity as well as reticulocyte synthesis and functions.
REFERENCES
- Ekor, M., 2014. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol., 4.
- Bandaranayake, W.M., 2006. Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs. In: Modern Phytomedicine: Turning Medicinal Plants into Drugs, Ahmad, I., F. Aqil and M. Owais (Eds.), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, ISBN: 9783527609987, pp: 25-57.
- Adel, A.A.M., A.M. Awad, H.H. Mohamed and S. Iryna, 2015. Chemical composition, physicochemical properties and fatty acid profile of tiger nut (Cyperus esculentus L) seed oil as affected by different preparation methods. Int. Food Res. J., 22: 1931-1938.
- Nina, G.C., A.F. Ogori, M. Ukeyima, L. Hleba and M. Císarová et al., 2019. Proximate, mineral and functional properties of tiger nut flour extracted from different tiger nuts cultivars. J. Microb. Biotechnol. Food Sci., 9: 653-656.
- Viuda-Martos, M., M.C. López-Marcos, J. Fernández-López, E. Sendra, J.H. López-Vargas and J.A. Pérez-Álvarez, 2010. Role of fiber in cardiovascular diseases: A review. Comp. Rev. Food Sci. Food Saf., 9: 240-258.
- Adejuyitan, J.A., 2011. Tigernut processing: Its food uses and health benefits. Am. J. Food Technol., 6: 197-201.
- Roselló-Soto, E., M.M. Poojary, F.J. Barba, J.M. Lorenzo, J. Mañes and J.C. Moltó, 2018. Tiger nut and its by-products valorization: From extraction of oil and valuable compounds to development of new healthy products. Innovative Food Sci. Emerging Technol., 45: 306-312.
- dos Santos Silveira Junior, J.F. and A. de Francisco, 2020. Unconventional food plants as an alternative in starch production. Cereal Foods World, 65.
- Jing, S., S. Wang, Q. Li, L. Zheng, L. Yue, S. Fan and G. Tao, 2016. Dynamic high pressure microfluidization-assisted extraction and bioactivities of Cyperus esculentus (C. esculentus L.) leaves flavonoids. Food Chem., 192: 319-327.
- Copley, M.S., P.J. Rose, A. Clapham, D.N. Edwards, M.C. Horton and R.P. Evershed, 2001. Detection of palm fruit lipids in archaeological pottery from Qasr Ibrim, Egyptian Nubia. Proc. R. Soc. Lond. B, 268: 593-597.
- Tengberg, M., 2012. Beginnings and early history of date palm garden cultivation in the Middle East. J. Arid Environ., 86: 139-147.
- Sirisena, S., K. Ng and S. Ajlouni, 2015. The emerging Australian date palm industry: Date fruit nutritional and bioactive compounds and valuable processing by-products. Comp. Rev. Food Sci. Food Saf., 14: 813-823.
- Ghnimi, S., S. Umer, A. Karim and A. Kamal-Eldin, 2017. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J., 6: 1-10.
- Al-Qarawi, A.A., H.M. Mousa, B.E.H. Ali, H. Abedl-Rahman and S.A. El-Mougy, 2004. Protective effect of extracts from dates (Phoenix dactylifera L.) on carbon tetrachloride-induced hepatotoxicity in rats. Int. J. Rev. Vet. Med., 2: 176-180.
- Perveen, K., N.A. Bokhari and D.A.W. Soliman, 2012. Antibacterial activity of Phoenix dactylifera L. leaf and pit extracts against selected gram negative and gram positive pathogenic bacteria. J. Med. Plants Res., 6: 296-300.
- Gangwar, A.K., A.K. Ghosh and V. Saxena, 2014. Standardization & antiulcer activity of Phoenix dactylifera Linn. leaves. World J. Pharm. Pharm. Sci., 3: 1164-1172.
- Ishurd, O. and J.F. Kennedy, 2005. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.). Carbohydr. Polym., 59: 531-535.
- Michael, H.N., J.Y. Salib and E.F. Eskander, 2013. Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytother. Res., 27: 699-704.
- Al-Qarawi, A.A., H. Abdel-Rahman, H.M. Mousa, B.H. Ali and S.A. El-Mougy, 2008. Nephroprotective action of Phoenix dactylifera. in gentamicin-induced nephrotoxicity. Pharm. Biol., 46: 227-230.
- El Arem, A., A. Thouri, M. Zekri, E.B. Saafi, F. Ghrairi, A. Zakhama and L. Achour, 2014. Nephroprotective effect of date fruit extract against dichloroacetic acid exposure in adult rats. Food Chem. Toxicol., 65: 177-184.
- Zhang, C.R., S.A. Aldosari, P.S.P.V. Vidyasagar, K.M. Nair and M.G. Nair, 2013. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J. Agric. Food Chem., 61: 5834-5840.
- Rahimi, S., H. Alaie, P. Reisi, Z. Siahmard, B. Zolfaghari and A. Pourshanazari, 2017. The effect of hydro-alcoholic of Phoenix dactylifera extract on sleep and EEG in rat. Avicenna J. Phytomed., 7: 511-518.
- Al-Kuran, O., L. Al-Mehaisen, H. Bawadi, S. Beitawi and Z. Amarin, 2011. The effect of late pregnancy consumption of date fruit on labour and delivery. J. Obstet. Gynaecol., 31: 29-31.
- Hussain, S.M., I.A. Awwad, M. Taha and O. Khan, 2015. A laboratory quest on use of date fruit (Phoenix dactylifera, L) extract in prevention of chemically induced memory deficit models in mice. Asian J. Biomed. Pharm. Sci., 5: 5-11.
- Nayar, N.M., 2017. Phylogeny. In: The Coconut: Phylogeny, Origins, and Spread, Nayar, N.M. (Ed.), Academic Press, Cambridge, Massachusetts, ISBN: 9780128097786 pp: 67-92.
- Perera, L., S.A.C.N. Perera, C.K. Bandaranayake and H.C. Harries, 2009. Coconut. In: Oil Crops, Vollmann, J. and I. Rajcan (Eds.), Springer, New York, ISBN: 978-0-387-77593-7, pp: 369-396.
- Roehl, E., 1996. Whole Food Facts: The Complete Reference Guide. Inner Traditions/Bear & Co., Rochester, Vermont, ISBN: 9780892816354, Pages: 296.
- Mat, K., Z. Abdul Kari, N.D. Rusli, H.C. Harun and L.S. Wei et al., 2022. Coconut palm: Food, feed, and nutraceutical properties. Animals, 12.
- da Silva Lima, R. and J.M. Block, 2019. Coconut oil: What do we really know about it so far? Food Qual. Saf., 3: 61-72.
- Wallace, T.C., 2019. Health effects of coconut oil-A narrative review of current evidence. J. Am. Coll. Nutr., 38: 97-107.
- Chinwong, S., D. Chinwong and A. Mangklabruks, 2017. Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: A randomized crossover trial. Evidence-Based Complementary Altern. Med., 2017.
- Lorke, D., 1983. A new approach to practical acute toxicity testing. Arch. Toxicol., 54: 275-287.
- Adefisayo, M.A., R.O. Akomolafe, O.S. Akinsomisoye, Q.K. Alabi, L. Ogundipe, J.G. Omole and K.P. Olamilosoye, 2018. Protective effects of methanol extract of Vernonia amygdalina (del.) leaf on aspirin-induced gastric ulceration and oxidative mucosal damage in a rat model of gastric injury. Dose-Response, 16.
- NRC, DELS, ILAR, CUGC and ULA, 2011. Guide for the Care and Use of Laboratory Animals. 8th Edn., National Academies Press, Washington, DC, ISBN: 9780309154000, Pages: 246.
- Odinga, T., C. Gabriel-Brisibe and B.W. Moore-Igwe, 2020. Evaluation of the effect of aqueous seed extract of Ricinodendron heudelotii on the blood electrolyte and hematological status of male Wistar albino rats. World J. Pharm. Life Sci., 6: 16-20.
- Wallis, D.J. and M.M. Hetherington, 2009. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite, 52: 355-362.
- Murphy, S.J., P. Smith, A.B. Shaivitz, M.I. Rossberg and P.D. Hurn, 2001. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. J. Am. Assoc. Lab. Anim. Sci., 40: 9-12.
- Fleming, C., H. Russcher, J. Lindemans and R. de Jonge, 2015. Clinical relevance and contemporary methods for counting blood cells in body fluids suspected of inflammatory disease. Clin. Chem. Lab. Med., 53: 1689-1706.
- Adenowo, A.F. and M.I. Kazeem, 2020. Tiger nut as a functional food, pharmacological and industrial agent: A mini review. Ann. Sci. Technol., 5: 31-38.
- Bamishaiye, E., N. Muhammad and O. Bamishaiye, 2009. Haematological parameters of albino rats fed on tiger nuts (Cyperus esculentus) tuber oil meal-based diet. Internet J. Nutr. Wellness, 10.
- Ezekwesili, C.N. and E.O. Iyeke, 2016. Effects of Cyperus esculentus (tigernut) milk on serum protein, haematological indices and antioxidant enzymes in Wistar rats. Bioscientist J., 4: 41-50.
- El-Abasy, M.A., D.H. Abdelhady, T. Kamel and M. Shukry, 2016. Ameliorative effect of coconut oil on hematological, immunological and serum biochemical parameters in experimentally infected rabbits. Alexandria. J. Vet. Sci., 50: 36-48.
- Onuh, S.N., E.O. Ukaejiofo, P.U. Achukwu, S.A. Ufelle, C.N. Okwuosa and C.J. Chukwuka, 2012. Haemopoietic activity and effect of crude fruit extract of Phoenix dactylifera on peripheral blood parameters. Int. J. Biol. Med. Res., 3: 1720-1723.
- Airaodion, A.I. and E.O. Ogbuagu, 2020. Consumption of tiger nut (Cyperus esculentus L.) improves haematopoiesis in Wistar rats. Int. J. Res. Rep. Hematol., 3: 13-19.
- Abdul Ameer, H.A. and N.F. Hassan, 2022. Investigation of hematological and biochemical effects of feeding date in the early morning on empty stomach vs. after nutrition on rabbits. Arch. Razi Inst., 77: 235-239.
- Tigner, A., S.A. Ibrahim and I.V. Murray, 2022. Histology, White Blood Cell. StatPearls Publishing, Treasure Island.
- Tall, A.R. and L. Yvan-Charvet, 2015. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol., 15: 104-116.
- Benavides, G.A., G.L. Squadrito, R.W. Mills, H.D. Patel and T.S. Isbell et al., 2007. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci., 104: 17977-17982.
- Rifkind, J.M., J.G. Mohanty and E. Nagababu, 2014. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol., 5.
- Gaertner, F. and S. Massberg, 2016. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Sem. Immunol., 28: 561-569.
How to Cite this paper?
APA-7 Style
Lemii,
B.C., Odinga,
T., Daka,
I.R., Gabriel-Brisibe,
C.U., Enebeli,
S.K., Austin-Asomeji,
I., Edward,
U.F. (2024). Effects of Synergistic Consumption of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on Hematological Indices in Male Rat Model. Asian Journal of Biological Sciences, 17(1), 156-168. https://doi.org/10.3923/ajbs.2024.156.168
ACS Style
Lemii,
B.C.; Odinga,
T.; Daka,
I.R.; Gabriel-Brisibe,
C.U.; Enebeli,
S.K.; Austin-Asomeji,
I.; Edward,
U.F. Effects of Synergistic Consumption of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on Hematological Indices in Male Rat Model. Asian J. Biol. Sci 2024, 17, 156-168. https://doi.org/10.3923/ajbs.2024.156.168
AMA Style
Lemii
BC, Odinga
T, Daka
IR, Gabriel-Brisibe
CU, Enebeli
SK, Austin-Asomeji
I, Edward
UF. Effects of Synergistic Consumption of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on Hematological Indices in Male Rat Model. Asian Journal of Biological Sciences. 2024; 17(1): 156-168. https://doi.org/10.3923/ajbs.2024.156.168
Chicago/Turabian Style
Lemii, Barizoge, Cletus, Tamuno-Boma Odinga, Iyaeneomi Ransome Daka, Christine Umanu Gabriel-Brisibe, Sarah Kelechi Enebeli, Iyingiala Austin-Asomeji, and Ucheawaji Felicia Edward.
2024. "Effects of Synergistic Consumption of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on Hematological Indices in Male Rat Model" Asian Journal of Biological Sciences 17, no. 1: 156-168. https://doi.org/10.3923/ajbs.2024.156.168

This work is licensed under a Creative Commons Attribution 4.0 International License.



