Metabolite Profiling and Cytotoxic Investigation of Senecio biafrae Oliv. & Hiern (Compositae) on Nauplii Brine Shrimps and Cancer Cell Lines
| Received 17 Mar, 2024 |
Accepted 15 Apr, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Senecio biafrae possesses various therapeutic properties including hepatoprotective and antioxidant potentials. However, there is no information regarding its cytotoxic activity. Hence, this study investigated the cytoactivities of methanolic leaf extract (MLE) and fractions of Senecio biafrae, profiled and elucidated their metabolites. Materials and Methods: Hydromethanolic extract of S. biafrae obtained by maceration was partitioned and subjected to cytotoxicity screening using brine shrimp, human cervical adenocarcinoma (HeLa) and Prostate Cancer Cell (PC-3) lines. Bioactive principles of most of the active fractions were profiled using GC-MS. The dichloromethane (DCM) fraction was purified using column and High-Performance Liquid Chromatography (HPLC) to afford compounds 1 and 2 identified by 1H and 13C, DEPT-90, DEPT-135, COSY, NOESY and EI-MS spectra. Results: The MLE and its fractions exhibited significant toxicity on nauplii brine shrimp, while the hexane fraction exhibited a pronounced cytoactivity on PC-3 cell line with 92.8% followed by DCM fraction. The GC-MS profiling of hexane and DCM fractions identified 17 and 6 constituents respectively, comprising saturated and unsaturated fatty acids, diterpenoidal alcohol, phytol and aromatic compounds. Hexadecanoic acid, cis, cis, cis-9, 12, 15-octadecatrienoic acid and phytol accounted for the highest constituents in both fractions. Structure elucidation confirmed compounds 1 and 2 as stigmasterol and ergosterol with cytoactivity which compared favourably with doxorubicin. Conclusion: The study revealed that S. biafrae contains cytoconstituents evidenced by proliferation inhibition of HeLa and PC-3 cell lines. The cytoactivities and bioconstituents indicate that S. biafrae has potential to be considered as a candidate for drug development that can manage cancer-related conditions. Further investigation into the specific bioactive compounds and their mechanisms of action could provide valuable insights into the therapeutic potential of S. biafrae in cancer treatment.
| Copyright © 2024 Akinola et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Man has depended on nature from time immemorial for basic needs of life like food, shelter, clothing, etc., with the majority of these natural products coming from plants, animals and minerals needed for treating human diseases1-3. Plants have become the bedrock of sophisticated traditional medicine for thousands of years and continue to provide mankind with new remedies4-6. This system continues today because of its biomedical benefits and cultural belief in many parts of the world making it a vital agent required for maintaining human health7. The World Health Organization (WHO) estimated that 80% of the population in developing countries continues to use traditional medicine to treat infectious diseases8. Medicinal plants are known to contain inherent active ingredients used to manage disease or relieve pain9. The use of traditional medicines and medicinal plants as therapeutic agents for the maintenance of good health in most developing countries has been widely observed10.
The growing interest in medicinal plants is influenced by the ever-rising cost of drugs available for maintenance of personal health and well-being of individuals as well as in bio-prospecting of new plant-derived drugs11. Continued interest in medicinal plants is due to several reasons like cultural faith in herbal medicine, easy accessibility and, low cost among others. Furthermore, consumption of medicinal plants has been linked to extraction and development of chemotherapeutic products from plants12. Examples are laxatives, blood thinners, antibiotics and anti-malaria medications having active ingredients from plant sources. The active constituents of plants commonly used in herbal medicines include taxol (from foxglove), vincristine (from yew) and morphine (from opium poppy)13. According to WHO, medicinal plants are the best sources for obtaining a variety of drugs, as they have proven to be good candidates for antimalarial, antibiotics and anticancer drugs14. As such, plants are often investigated to better understand their properties, safety andefficacy15. The use and efficacy of medicinal plants in the management of various human pathological conditions in Africa predate the introduction and use of orthodox drugs16.
There is a resurgence of interest in the pharmacological efficacy of medicinal plants in the management of various human carcinomas and other pathologies, especially in the face of increasing failure and toxicity associated with the use of some anticancer agents17. Numerous bioactive compounds or their derivative complexes from medicinal plants have been reported for chemopreventive and anticancer activity18. Senecio biafrae Oliv. & Hiern (Compositae), commonly known as a life root plant, is one of the most important medicinal plants with edible leaves and stems, widely consumed as vegetables among the Yoruba tribe of South-Western, Nigeria19.
Phytochemical investigations of Senecio biafrae revealed the presence of phytometabolites belonging to different classes of chemical diversities such as terpenoids, flavonoids, saponins, alkaloids, anthocyanins, coumarins, glycosides and dihydroisocoumarins20. However, plants of the genus Senecio contained active compounds such as pyrrolizidines alkaloids21 and androgenic steroids22. Senecio biafrae has been reported to be a good source of protein having high levels of essential amino acids and vitamins A, C and E and has been recommended as a food supplement23. The leaf of S. biafrae is ethnomedicinally used for the treatment of wounds, sore eyes, infertility, rheumatism, pulmonary defects, heart problems, cough and diabetes and promotion of milk secretion in lactating mothers24,25. Besides its ethnomedicinal values, species of S. biafrae exhibit a wide range of biological activities, such as antitrypanosomal and hepatoprotective activities26,27, anti-inflammatory28, vasodilatory effect29, antimicrobial and, antioxidant30. A few species of Senecio have been reported for their Cytotoxic activities3,28,31.
There is a paucity of reports on the cytotoxic activity of the S. biafrae. Hence, this study investigated the cytotoxic activity of Senecio biafrae leaf extract and its fractions on nauplii brine shrimp, human cervical adenocarcinoma (HeLa) and Prostate Cancer Cell (PC-3) lines and carried out the chemical profiling, isolation, purification and structure elucidation of the active chemical principles on the two most active fractions.
MATERIALS AND METHODS
The study was carried out from January 2019 to July 2021 at Obafemi Awolowo University, Ile- Ife, Nigeria and H.E.J. Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, Pakistan.
General experimental procedure: Proton 1H-NMR and carbon 13C-NMR experiments were acquired on Bruker (Avance NEO) spectrometer operating at 500 MHz and 600 MHz, respectively, with TMS (tetramethylsilane) as internal standard. Chemical shifts (δ) were reported in parts per million (ppm) and coupling constant (J) in Hz. All spectra were recorded in Deuterated Methanol (CD3OD) and Deuterated Chloroform (CDCl3) from Sigma-Aldrich Chemie GmbH Germany. The NMR and MS facility was accessed from Hussein Ebrahim Jamal Research Institute of Chemistry International Center for Chemical and Biological Sciences (ICCBS) University of Karachi, Pakistan. All spectra were processed using MestReNova-9.0.1 software. The ES-MS was conducted on a JEOL 600H-1 spectrometer (Thermo Scientific, USA). Data were processed by Xcalibur Software. For column chromatography, Silica gel 60 (Merck Kiesel gel 60, 70-230 mesh size) was used as a solid matrix.
Recycling preparative HPLC was performed using Shimadzu LC-908W system equipped with BG-42-10 degassing unit, a RI-700 LA detector, SIL-20ACHT autosamplers and a JAIGEL- SIL column (20×250 mm) using Lab Solution software system. The mobile phase was composed of ethyl acetate (solvent B) and hexane. TLC was carried out on pre-coated silica gel 60 plates (PF254; Merck). Cytotoxicity assay was measured on an Infinite 200 Pro-TECAN plate reader.
Plant collection: Senecio biafrae leaves were harvested in January 2019 from Iperindo, Nigeria (Latitude 07°29’40”; Longitude E04°49’26”). Voucher specimen of the collected leaves was deposited at the IFE Botanical Herbarium and assigned number (IFE-17936), Obafemi Awolowo University. The botanically accepted nomenclature and authority of the plant were confirmed by visiting the World floraonline database. The leaves were dried and ground into powder using a mechanical grinder (SR-14733, Marlex, Daman and Diu, India).
Preparation of extract and solvent partitioning: The powdered leaf (2 kg) was twice macerated with 10 L of 80:20 (v/v) MeOH:H2O for 2 days at room temperature while shaking on a Hoover MK IV motor shaker as described by Adekola et al.32, Okoro et al.33. This extraction method was used to preserve the chemical integrity of the metabolites as they solubilize into the extracting solvent without degradation. The combined liquid extract obtained was filtered using Whatman No. 1 filter paper and then evaporated under reduced pressure in a rotary evaporator (BUCHI, Rotavapor R-210) at 40°C to obtain a dry crude hydromethanolic extract (45 g), which was reconstituted in distilled H2O (1:6) and subjected to liquid-liquid partitioning using n-hexane, dichloromethane (DCM), ethyl acetate (EA) and butanol according to Oriyomi et al.34 to afford hexane fraction, DCM fraction, EA fraction, butanol fraction and, aqueous fraction. These fractions were separately concentrated in vacuo at 40°C using a rotary evaporator (BUCHI, Rotavapor R-210, Philadelphia, USA) except butanol and aqueous fractions which were freeze-dried. The extract and its fractions obtained were subjected to cytotoxic testing to locate the fraction(s) with the most cytotoxic activity. Following the cytotoxic testing, n-hexane and dichloromethane fractions exhibited the most cytotoxic activity and were further analyzed.
Metabolite profiling of active fractions: The profiling of the active fractions was performed on a Gas Chromatography-Mass Spectrometer (GCMS). Hexane and dichloromethane fractions were separately loaded on AT-5MS fused silica capillary column 30 m×0.25 mm, 0.25 μm film thickness equipped with an Agilent 5975C model gas chromatograph interfaced with inert XL EI/CI MSD fitted with a triple-axis detector source (Agilent Technologies, Santa Clara, California, USA). A 1:30 split injection ratio was used, while injection pot temperature was maintained at 270°C and column temperature at 60°C, with a steady increase of 3°C every minute to 270°C for a run-time of 30 min. The carrier gas (helium) was set at a flow rate of 1 mL per minute, while 70 eV EIMS with an ions source temperature of 230°C, quadrupole temperature of 150°C, mass range (m/z) of 29-550 with a scan rate of 6.35 scan/s and electron multiplier voltage 1341 V. Retention time of components and mass spectra matching with NIST MS library 2009 were used for the identification. The concentration of identified components was calculated using area normalization over flame ionization detector response.
Fractionation of dichloromethane fraction on silica gel column: This was carried out on an open column with silica gel 200-400 mesh as a stationary phase. The column was eluted with n-hexane and increasing gradient of ethyl acetate and methanol respectively to yield 13 subfractions A-M. Sephadex LH 20 column was used to further fractionate subfraction G using isocratic elution with CH2Cl2:MeOH (9:1 v/v) to give sub-fractions coded G1-G30 and analyzed by TLC. Sub-fractions G6-G15 were selected and fractionated using silica gel column to afford a white powder as precipitate, which was filtered off and crystallized with CHCl3. This gave Compound 1 sub-fraction H was similarly treated on an open column eluted with CH2Cl2 and gradient with EtOAc and MeOH to give sub-fractions H1-H10. Sub-fractions H6-H10 were combined, dried and chromatographed on a column to yield an off-white powder, which was dissolved in CH2Cl2 and purified on sephadex LH 20 column to give Compound 2 which was recrystallized from CHCl3 to give a white solid.
Brine shrimp lethality assay: Brine shrimp lethality assay was carried out on S. biafrae extract/fractions as described by Meyer et al.35. Ten shrimp nauplii were exposed into three replicates of each concentration (10, 100 and 1000 μg/mL) as described by Nazeer et al.36 of the plant extract/fractions for 24 hrs in sea-water solutions (10 mL) with one percent dimethyl sulfoxide. Distilled water was used to conduct control blank. After 24 hrs the number of mobile brine shrimp larvae was counted and percentage of death was calculated using the equation below. The LC50 (Median Lethal Concentration) values were determined from a calibration curve by linear regression obtained by plotting the concentration against the death percentage by Waghulde et al.37:
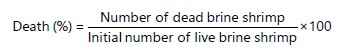 |
Cytotoxicity assay: Cytotoxic activity of extract, fractions and isolated compound of S. biafrae plant, was carried out in 96-well flat-bottomed microplates using standard MTT [3-(4,5-dimethyl thiazole-2-yl)-2,5-diphenyl-tetrazolium bromide] colorimetric assay38. Two cancer cell lines; human cervical adenocarcinoma (HeLa) and human epithelia prostate cancer (PC-3) were used in this experiment. Briefly, the concentration of HeLa cells and PC-3 cells was adjusted to 6×104 cells/mL in RPMI-1640 and Dulbecco Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. The cell lines were maintained at 37°C in a 5% CO2 atmosphere.
For this assay, 100 μL cell suspensions (6×104 cells/mL) were seeded in round bottom 96 well-plate at the density of 10,000 cells/well and incubated overnight at 37°C in 5% CO2. After overnight incubation, the medium was carefully removed and a fresh medium of 200 μL was added with different concentrations (1, 3 and 30 ug/mL) of the extract, fractions and isolated compounds of the plant. After treatment with the plant extracts, the cells were incubated for 48 hrs at 37°C in 5% CO2.
After 48 hrs, 50 μL of sterilized MTT (0.5 mg/mL) in phosphate-buffered saline (PBS) was added to each well and incubated for four hours. The medium with MTT was removed and the formed formazan crystals
were solubilized by the addition of 100 μL of DMSO and the absorbance was read at 570 nm using a microplate reader (Spectra Max Plus, Molecular Devices, California, USA). Doxorubicin (standard drug) serves as a positive control, while DMSO is used as a negative control. The percent inhibition was calculated by using the following formula39:
Where:
| B | = | Mean OD of test compound | |
| A | = | Mean OD of negative control | |
| C | = | Mean OD of positive control |
The experiment was repeated five (5) times.
Statistical analyses: All data obtained for fractions on nauplii mortality and cytotoxicity on human cervical adenocarcinoma (HeLa) and prostate cancer cell (PC-3) lines studies were subjected to GraphPad Prism (Version 5.0) for statistical analyses. The concentration of fractions that resulted in 50% lethality (LC50) of brine shrimp nauplii and cell lines was estimated by plotting a linear regression of percentage inhibition of the subject against a concentration of the fraction. Results were expressed as inhibition (%) and median lethal concentration (LC50).
RESULTS AND DISCUSSION
Brine shrimp lethality of S. biafrae plant: The result of brine shrimp lethality of S. biafrae extract and fractions is presented in Table 1. The result showed that the LC50 value of various fractions and extracts was found to be less than 1000 μg/mL. Etoposide (standard) and butanol fraction greater toxic effect (LC50 value of <500 μg/mL) than that of methanolic extract (LC50 value of 571.32 μg/mL), ethyl acetate (EA) fraction (LC50 value of 572.73 μg/mL) and, aqueous fraction (LC50 value of 576.95 μg/mL), while hexane fraction and dichloromethane (DCM) fraction had weak toxic properties (LC50 value of >500 μg/mL). The order of the LC50 value of S. biafrae extract and fractions was found to be butanol fraction Cytotoxicity of S. biafrae: The antiproliferative activities of S. biafrae extract, fractions and isolated compounds are presented in Table 2. The plant extract, fractions and isolated compounds were screened for their cytotoxicity against human epithelial Prostate Cancer (PC-3) cells and human cervical adenocarcinoma (HeLa) cell line at 30 μg/mL concentration. The results showed that hexane fraction actively inhibited human epithelial Prostate Cancer (PC-3) proliferation by 92.80%. Crude extract, DCM, ethyl acetate, butanol, aqueous fractions and isolated compounds were found to be inactive against human epithelial Prostate Cancer (PC-3) cell line. Compound 1 also showed a moderate inhibition against human cervical adenocarcinoma (HeLa) proliferation by 45.90% but crude extract, hexane, DCM, ethyl acetate, butanol, aqueous fractions and compound 2 were found to be inactive against human cervical adenocarcinoma (HeLa) cell line. Metabolite profiling of hexane and dichloromethane fractions: Seventeen chemical constituents were identified in the hexane-soluble fraction, while six constituents were identified in the dichloromethane-soluble fractions. Figure 1 and 2 show the total ion chromatograms of hexane and dichloromethane fractions, respectively, while Table 3 and 4 show the chemical constituents identified. n-Hexadecanoic acid (35.90%), Cis, Cis, Cis-9, 12, 15-Octadecatrienoic acid, (21.85%), Phytol (9.40%), Methyl n-Hexadecanoate, (7.38%), Cis, Cis-9, 12-Octadecadienoic acid (6.08%) and Methyl-Cis, Cis, Cis-9,12,15-Octadecatrienoate, (5.97%) accounted for more than 80% of the total chemical components, while the minor compounds identified were Methyl-Cis, Cis-9,12-Octadecadienoate, (2.80%), Octadecanoic acid (1.70%), 6,10,14-Trimethyl-2-Pentadecanone, (1.50%), Methyl Octadecanoate, (0.90%), 3,7,11,15-Tetramethyl-2-Hexadecen-1-ol (0.70%), Diisooctyl-1,2-Benzenedicarboxylate, (0.60%), 3,7,11,15-Tetramethyl-2-Hexadecen-1-ol (0.30%), 2H-Pyran-2-one, Tetrahydro-6-Nonyl (0.30%), Methyl Eicosanoate (0.20%), 2,6-Bis(1,1-Dimethylethy)-1,4-Benzenediol (0.10%) and 3-Butoxy-1,1,1,5,5,5-Hexamethyl-3-(Trimethylsiloxy)Trisiloxane (0.10%). Structure elucidation of Compounds 1 and 2: Compounds 1 and 2 were found to be a steroidal compound. Identification of 1 and 2 using spectroscopic techniques revealed a steroidal nucleus in both compounds. The molecular ion peak [M]+ of 1 was observed at m/z 421.2 which is also the peak with highest intensity (base peak). Other peaks observed in the mass spectrum (fragment ions) were at m/z 396.2 [M-CH4]+ which is due to loss of methane and m/z 381.2 [M-C2H7]+. The mass of 421.2 is consistent with molecular formula, C29H48O of Stigmasterol (24-(S)-Stigmasta-5,22-dien-3β-ol), while the [M]+ of 2 was observed at m/z 396.4 with 100% relative abundance. The daughter ions of the [M]+ at m/z 382 due to removal of CH2 [M-CH2]+, m/z 255 (21), m/z 147 (19). The molecular mass of m/z 396.4 is consistent with the molecular formula C28H44O for Ergosterol (22E)-Ergosta-5,7,22-trien-3β-ol. The above mass spectrum information differentiates 2 from 1. From the 1H-NMR of 1, three methine signals at δH 5.34 (1H, s, H-6); 5.17 (1H, d, 8.2 Hz, H-22) and 5.00 (1H, d, 8.2 Hz, H-23) were seen in the olefinic region. The 3JHH value of 8.2 Hz indicated the cis orientation of the olefinic double bond between C-22 and C-23, which was confirmed by the NOESY spectrum. Similarly, the 1H-NMR spectrum of 2 showed spectroscopic information similar to 1 in the olefinic region showing four methine signals at δH 5.27 (1H, d, H-6), 5.13 (1H, m, H-7),
5.00 (1H, d, 15.1 Hz, H-22) and 4.97 (1H, m, H-23). The DEPT-90 experiment of 1 and 2 confirmed the signal at δH 3.45 (1H, m, H-3) with δc 71.8 and δH 3.27 (1H, m, H-3) and δc 69.9, respectively to be an oxymethine carbon (CH-O) in both Compound 1 and 2. Analysis of 13C-NMR and DEPT-90 spectra of 1 showed signals for four olefinic carbons at δc 140.8 (C-5), 138.3 (CH-23), 129.3 (C-22) and 121.7 (CH-6), while DEPT-135 showed that δc 140.8 is a quaternary olefinic carbon. However, for Compound 2, six olefinic carbons signals comprising two quartenary carbon signals at δc 140.1 (C-8) and 138.7 (C-5), with four methine signals included δc 130.3 (CH-22), 129.4 (CH-23), 121.9 (CH-6) and 100.9 (CH-7). The DEPT-135 information of 2 further differentiates it from 1. The upfield region of the spectrum of Compounds 1 and 2 showed similar signal pattern, comprising many alkyl signals ranging from δH 0.5-2.5 ppm in the 1H-NMR and δc 10-60 in the 13C-NMR, this confirmed the stigmastane unit of 1 and ergostane nucleus of 2. The spectra information obtained above on 1 and 2 agree with the literature data on Stigmasterol and Ergosterol. The lethality of the test sample like brine shrimp (Artemia sp.) has been utilised by many researchers and has proven to be useful tool in screening various phytochemicals found in various bioactivities. However, brine shrimp lethality assay is a cheap techniques and are capable of detecting a spectrum of bioactivity present in plant extracts37. In this study, it was observed that methanolic extract and fractions of S. biafrae leaves exhibited brine shrimp cytotoxic activity with significant LC50 value. The present study revealed that the extent of lethality was directly proportional to the concentration of the methanolic crude extract/fractions of the plant, that is, maximum mortalities were observed at a concentration of 1000 μg/mL and the least mortality at 10 μg/mL concentration. Based on the results, the brine shrimp lethality of methanolic extract and fractions of S. biafrae were found to be concentration dependents. Hence, hexane and dichloromethane fractions of the plant demonstrated relatively lower cytotoxic effects compared to the other fractions and extract tested, as well as the standard drug (etoposide). This suggests that these particular fractions may have safer properties and could potentially be explored further for their therapeutic applications with reduced risk of adverse effects40,41. According to Meyer et al.35, substances with LC50 value <1000 μg/mL are toxic while ones with values >1000 μg/mL are non-toxic. However, further specific bioassays are needed to fully understand the safety and potential benefits of these fractions in the context of drug development and cancer management.
The toxicity observed by the n-hexane fraction against the brine shrimp suggests its potential as a bioactive compound with cytotoxic effects, as also confirmed by its potent inhibitory activity against prostate cancer cell. Thus, active inhibitory activity showed by the n-hexane fraction against prostate cancer cells could be as a result of phytochemicals that are present in the fraction and their synergistic interaction42. The GC-MS analysis revealed the presence of n-Hexadecanoic acid (fatty acid), (Z,Z,Z)-9,12,15-Octadecatrienoic acid, (fatty acid) and phytol (diterpene) as most abundant phytoconstituents presence in the fraction. The n-hexadecanoic acid and phytol have been reported to possess cytotoxic activity43,44. According to Ravi and Krishnan45 n-hexadecanoic acid exerted its cytotoxic activity against human fibroblast cells by inhibiting DNA topoisomerase-I thereby preventing proliferation of cells. The ability of the n-hexane fraction to inhibit proliferation of the prostate cancer cell cannot be explained only in terms of the n-Hexadecanoic acid and phytol content. Other compounds present may have a synergistic effect on the activity of these compounds, therefore n-hexane fraction of S. biafrae can further undergo investigations. This present finding revealed that, among all the fractions and extract of S. biafrae leaf, n-hexane fraction possesses selective cytotoxicity, which was found to be more effective in prostate cell line and less effective in HeLa cell line. This significant inhibitory activity observed in hexane fraction against PC-3 cell line is in line with the previous studies on other Senecio species. According to Loizzo et al.46, the n-hexane extract of S. ambiguus exhibited a strong inhibitory activity against prostate carcinoma cell line, a similar result to the n-hexane fraction of S. biafrae against prostate cancer cell line indicating that all Senecio species might have some activities in common. Compound 1 was found to exhibit moderate inhibition (%) on the proliferation of HeLa and PC-3 cell lines respectively compared to the standard drug used. Compound 1 exhibited a better cytotoxic activity than Compound 2. Bioactive profile may aid to correlate the responsible key compounds required for distinct biological activities, as well as in revealing underlying mechanisms. In this study, six bioactive constituents were present in DCMF while six major compounds were present in hexane fraction of S. biafrae plant. Most of the major six compounds were fatty acids except phytol and benzene dicarboxylate esters. These six compounds were reported to have several biological activities. The n-Hexadecanoic acid which is the most abundant phytoconstituents present in hexane fraction had been reported to exert several biological activities including anti-cancerous, anti-inflammatory, antioxidant and anti-androgenic pharmacological properties47. This was followed by unsaturated fatty acid cis, cis, cis-9,12,15-Octadecatrienoic acid and phytol. Studies have shown that phytol, a diterpene possess strong cytotoxic effects, antioxidant and anti-inflammatory activities48,49. In this study, two phytosterols were isolated from the dichloromethane fraction of S. biafrae plant. The isolated phytochemicals were found as white amorphous solid compounds which gave a positive test to both Liebermann-burchard and Salkowski reagents, indicating the presence of steroidal structures. The identification of stigmasterol and ergosterol in the S. biafrae fraction may contribute to the observed cytotoxic activity. Phytosterols, as steroidal compounds, are vital components of plant cell membranes. They have a wide range of functions that are essential for plant growth and development50,51. Since phytosterol are essential fatty acids, they need to be included in diet52. Eating foods or supplements enriched with phytosterols, help to reduce intake of cholesterol in intestine leading to low-density lipoproteins (LDL) in the blood53. Stigmasterol a steroid derivative which is present in many naturally occurring plants, such as vegetables, legumes, nuts, seeds, tobacco, as well as in the fat and oils of soybean, Calabar bean and rape seed43,44. It also serves as a precursor for the synthesis of various human steroid hormones like androgen, estrogen, corticoids, progesterone, etc. It is used as raw materials for the synthesis of vitamin D3, it is also used as food additives in the food and beverages industries54. Pharmacologically, stigmasterol possess activities like antioxidant55,56, anti-inflammatory57,58, anti-osteoarthritis, hypoglycemic and anti-hypercholestrolemic59,60. It possesses anti-cancer activities in many organs of the body such as the breast, liver etcetra61, by promoting apoptosis, inhibiting proliferation and metastasis by regulating the PI3K/Akt signaling pathway and exhibiting a modulatory effect on cyclin proteins and cyclin-dependent kinase62. In this study, the cytotoxicity of the stigmasterol has been examined. It has been observed that, the stigmasterol exhibited considerable cytotoxic effects on the human epithelial adenocarcinoma cell than prostate cancer cells at the concentration of 30 μg/mL. The differences in cytotoxic effects observed on human epithelial adenocarcinoma cells and prostate cancer cells when exposed to stigmasterol could be due to a combination of cell type-specific factors, including receptor expression, signaling pathways, metabolism, cell cycle regulation, defense mechanisms and genetic variations63,64. Based on this study and other previous studies on the cytotoxicity of stigmasterol, it can be concluded that stigmasterol exhibited more effective cytotoxicity on other cancer cells than the PC-3 cell line. Ergosterol is a sterol compound that is usually found in fungal cell membranes where it plays a role that is analogous to cholesterol in animal cell membranes65. It is an essential component required for maintaining the integrity and fluidity of the cell membrane. Ergosterol also serves as a precursor for synthesizing other important molecules66,67. The pharmacological activities of ergosterol include antioxidant, anticoagulant, anti-inflammatory, anticancer and defense agent68,69. In this study, ergosterol exhibited a low cytotoxic effect against PC-3 and HeLa cell lines. The PC-3 and HeLa cells could have exhibited resistance against ergosterol-induced cytotoxicity70 or provided a microenvironment that protected them from the cytotoxic effects of ergosterol.
This work provides experimental evidence that S. biafrae leaf fraction contains metabolite that exhibited cytotoxic potential without toxic effects on normal cells. Methanolic extract/fractions of S. biafrae leaf exhibited cytotoxic activity when tested against brine shrimp larvae and were considered as containing active or potent components. The antiproliferative activity observed in hexane fraction of S. biafrae leaf against human epithelia Prostrate Cancer (PC-3) may be due to the synergistic interaction of bioactive compounds present in the fraction. Two phytosterol compounds: Stigmasterol and Ergosterol were isolated from dichloromethane fraction of S. biafrae. Compound 1 (Stigmasterol) showed a moderate activity on the HeLa cell, while other fractions and Compound 2 (Ergosterol) exhibited a weak activity.
Cancer remains one of the major causes of mortality worldwide and the current cancer treatment is a combination of surgery, radiation and chemotherapy. However, available anticancer drugs are associated with adverse side effects damaging vital human cells, tissues and organs. Hence, numerous bioactive compounds and their derivative complexes from medicinal plants have been reported for chemopreventive and anticancer activity. This study, therefore investigated the cytotoxic activities and profiling of Senecio biafrae leaf extract and its fractions.
Akinola Funke Toyin acknowledges the World Academy of Sciences for the Advancement of Science in Developing Countries (TWAS), Trieste, Italy for ICCBS-TWAS Fellowship Award with FR number: 3240305613 at the H.E.J. Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, Pakistan. The authors are also grateful to the MS and NMR Unit of H.E.J Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, Pakistan for recording the GC-MS, NMR and MS spectrum of hexane fraction and isolated compounds, respectively.
Table 1:
Number of brine shrimp nauplii that survived after treating with Senecio biafrae extract and fractions and the percentage mortality
Number of surviving nauplii (after 24 hrs)
Plant extract
Concentration
(μg/mL)R1
R2
R3
Total number of
nauplii survivorMortality
(%)LC50
(μg/mL)
Control
10
10
10
10
30
0
0
(distilled water)
100
10
10
10
30
0
1000
10
10
10
30
0
Standard
10
10
9
9
28
6.7
465.6
(etoposide)
100
7
6
6
19
36.7
1000
1
1
0
2
93.3
Senecio biafrae
10
10
10
9
29
3.3
571.3
(methanolic extract)
100
10
10
9
29
3.3
1000
9
9
9
27
10
Senecio biafrae
10
10
10
10
30
0
610.7
(hexane fraction)
100
10
10
9
29
3.3
1000
3
3
3
9
70
Senecio biafrae
10
10
10
10
30
0
610.7
(DCM fraction)
100
10
9
9
28
6.6
1000
0
0
0
0
100
Senecio biafrae
10
10
10
10
30
0
572.7
(EA fraction)
100
10
9
9
28
6.6
1000
9
9
9
27
10
Senecio biafrae
10
9
9
9
27
10
483.9
(butanol fraction)
100
8
8
8
24
20
1000
8
8
8
24
20
Senecio biafrae
10
10
10
10
30
0
576.9
(aqueous fraction)
100
10
9
9
28
6.6
1000
8
8
8
24
20
DCM: Dichloromethane fraction, EA: Ethyl acetate, LC50: Median lethal concentration and Values represent Mean±SEM (n = 3)
Table 2:
Antiproliferative activities of methanolic crude extract, fractions and compounds 1 and 2 of S. biafrae leaves
Inhibition (%) at 30 μg/mL
Fraction/drug
PC-3
HeLa
Crude extract
0.7
6.9
Hexane
92.8
20.2
DCM
17.1
6.8
EA
-17.7
-8.4
Butanol
-24.1
6.1
Aqueous
-12
-0.6
Compound 1
21.9
45.9
Compound 2
13.4
15.2
Doxorubicin
89.9
101.2
DMSO
0.0
0.0
DCM: Dichloromethane, EA: Ethyl Acetate, DMSO: Dimethyl sulfoxide and Values represent Mean±SEM (n = 5)

Fig. 1:
GC-MS chromatogram of hexane fraction
*Prominent in the hexane-soluble fraction of Senecio biafrae leaf extract

Fig. 2:
GC-MS chromatogram of dichloromethane fraction
*Prominent in the dichloromethane fraction of Senecio biafrae leaf extract
•
Compound 1 (Stigmasterol) (Fig. 3), is an off-white crystalline powder and 73 mg yield
•
1H-NMR (500 MHz, CDCl3) and 13C-NMR (600 MHz, CDCl3) (Table 5)
•
EI-MS [M]+ m/z 421.2 (100) calculated for C29H48O, [M-CH4]+m/z 396.2 (43), [M-C2H7]+ m/z 381.2, 300.1 (41), 271.1 (54), 255.1 (78), 159.0 (42), 83 (40), 69 (27), 55.0 (41) 43 (20)
•
Compound 2 (Ergosterol) (Fig. 3), a white powder, 45 mg yield
•
1H-NMR (500 MHz, CDCl3+CD3OD) and 13C-NMR (600 MHz, CDCl3+CD3OD) (Table 6)
•
EI-MS [M]+ m/z 396.4 (100) calculated for C28H44O, [M-CH2]+ m/z 382 (23), 255 (21), 147 (19)
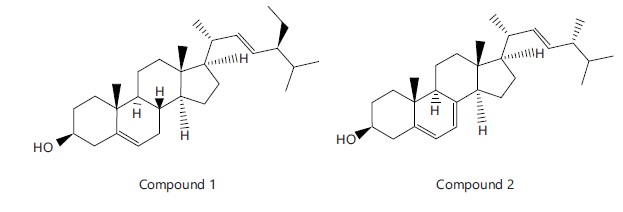
Fig. 3:
Structures of Compounds 1 and 2
Table 3:
Constituents of hexane-soluble fraction of S. biafrae leaf extract
S/N
Compounds
Formula
MW
RT (min)
Area (%)
1
6,10,14-Trimethyl-2-Pentadecanone
C18H36O
268
35
1.5
2
Methyl n-Hexadecanoate
C17H34O2
270
41
7.38
3
n-Hexadecanoic acid
C16H32O2
256
43.9
35.9
4
Methyl-Cis, Cis-9,12-Octadecadienoate
C19H34O2
294
48.7
2.8
5
Methyl-Cis, Cis, Cis-9,12,15-Octadecatrienoate
C19H32O2
292
48.9
5.97
6
Phytol
C20H40O
296
49.3
9.4
7
Methyl Octadecanoate
C19H38O2
298
49.5
0.9
8
Methyl Cis, Cis-9,12-Octadecadienoate
C18H32O2
280
49.7
6.08
9
Cis, Cis, Cis-9,12,15-Octadecatrienoic acid
C18H30O2
278
49.9
21.85
10
Octadecanoic acid
C18H36O2
284
50.3
1.70
11
3,7,11,15-Tetramethyl-2-Hexadecen-1-ol
C20H40O
296
51.5
0.70
12
Methyl Eicosanoate acid
C21H42O2
326
53.4
0.20
13
Tetrahydro-6-Nonyl-2H-Pyran-2-one
C14H26O2
226
53.9
0.30
14
3,7,11,15-Tetramethyl-2-Hexadecen-1-ol
C20H40O
296
54.4
0.40
15
3-Butoxy-1,1,1,5,5,5-Hexamethyl-3-(Trimethylsiloxy)Trisiloxane
C13H36O4Si4
368
56.2
0.10
16
1,2-Diisooctyl-Benzenedicarboxylate
C24H38O4
390
56.6
0.60
17
2,6-Bis(1,1-Dimethylethy)-1,4-Benzenediol
C14H22O2
222
56.9
0.10
MW: Molecular weight and RT: Retention time
Table 4:
Constituents identified in the dichloromethane fraction of S. biafrae
S/N
Compounds
Formula
MW
RT (min)
Area (%)
1
3,7,11,15-Tetramethyl-2-Hexadecen-1-ol
C20H40O
296
34.6
4.7
2
n-Hexadecanoic acid
C16H32O2
256
43.6
17.2
3
Phytol
C20H40O
296
49.3
12.7
4
Cis, Cis-9,12-Octadecadienoic acid
C18H32O2
280
49.7
9.5
5
Methyl-Cis, Cis, Cis-9,12,15-Octadecatrienoate
C19H32O2
292
49.9
52.5
6
Cis, Cis, Cis-9,12,15-Octadecatrienoic acid
C18H30O2
278
50.3
3.5
Table 5:
Spectroscopic data of Compound 1 (CDCl3)
Compound 1
Stigmasterol39
Position
δH (ppm), multiplicity, J value (Hz)
δC (ppm)
δH (ppm)
δC (ppm)
1
37.3
37.6
2
31.9
32.1
3
3.45, 1H, m
71.8
3.51, 1H, tdd
72.1
4
42.4
42.4
5
140.7
141.1
6
5.34,1H, s,
121.7
5.31, 1H, t
121.8
7
31.6
31.8
8
30.9
31.8
9
50.2
50.2
10
36.5
36.6
11
19.7
21.5
12
39.7
39.9
13
42.5
42.4
14
56.9
56.8
15
24.3
24.4
16
28.9
29.3
17
56.7
56.2
18
1.13, 3H, s
12.4
1.03, 3H, s
12.2
19
0.68, 3H, s
18.9
0.71, 3H, s
18.9
20
39.7
40.6
21
21.3
0.91, 3H, d, 6.2 Hz
21.7
22
5.17, 1H, d, 8.2 Hz
138.3
5.14, 1H, m
138.7
23
5.00, 1H, d, 8.5 Hz
129.3
4.98, 1H, m
129.6
24
0.84, 1H, m
45.8
46.1
25
25.7
25.4
26
0.89, 3H, m
12.3
0.83, 3H, t
12.1
27
29.1
29.6
28
0.81, 3H, d,6.8 Hz
19.9
0.82, 3H, d, 6.6 Hz
20.2
29
0.80, 3H, d, 6.8 Hz
19.1
0.80, 3H, d, 6.6 Hz
s, d, m, J means singlet, doublet, multiplets and coupling constant, respectively
Table 6:
Spectroscopic data of Compound 2 (CDCl3+CD3OD)
Compound 1
Ergosterol40
Position
δH (ppm), multiplicity, J value (Hz)
δC (ppm)
δH (ppm)
δC (ppm)
1
38.4
38.4
2
31.7
31.9
3
3.27, 1H, m
69.9
3.64, 1H, m
70.5
4
39.5
42.8
5
138.7
139.9
6
5.27,1H, s,
121.9
5.58, 1H, dd, 5.5,3.0 Hz
119.6
7
5.13, 1H, m,
100.9
5.38, 1H, dd, 5.4,2.9Hz
116.3
8
140.1
141.4
9
45.6
46.2
10
37
37.1
11
19.5
21.1
12
40
39.1
13
39.5
42.9
14
55.8
54.6
15
22.3
22.9
16
28.9
28.3
17
56.5
55.7
18
11.7
0.95, 3H, s
12.1
19
15.7
0.65, 3H, s
16.3
20
40.4
40.3
21
1.01, 3H, s
20.8
1.04, 3H, d, 6.6 Hz
21.1
22
5.00, 1H, d, 15.1 Hz
130.3
5.20, 1H, m
135.6
23
4.97, 1H, m
129.4
5.21, 1H, m
131.9
24
0.84, 1H, m
43.2
42.9
25
33.7
33.1
26
0.88, 3H, m
19
0.84, 3H, d, 6.7 Hz
19.9
27
0.86, 3H, m
18.7
0.82, 3H, d, 6.7 Hz
19.7
28
0.93, 3H, m
18.5
0.82, 3H, d, 6.6 Hz
17.6
s, m, d, J means singlet, multiplets, doublets and coupling constant, respectively
CONCLUSION
SIGNIFICANCE STATEMENT
ACKNOWLEDGMENTS
REFERENCES
How to Cite this paper?
APA-7 Style
Akinola,
F.T., Oriyomi,
V.O., Ogundele,
S.B., Adekola,
M.B., Agbedahunsi,
J.M., Babalola,
O.O. (2024). Metabolite Profiling and Cytotoxic Investigation of Senecio biafrae Oliv. & Hiern (Compositae) on Nauplii Brine Shrimps and Cancer Cell Lines. Asian Journal of Biological Sciences, 17(3), 291-306. https://doi.org/10.3923/ajbs.2024.291.306
ACS Style
Akinola,
F.T.; Oriyomi,
V.O.; Ogundele,
S.B.; Adekola,
M.B.; Agbedahunsi,
J.M.; Babalola,
O.O. Metabolite Profiling and Cytotoxic Investigation of Senecio biafrae Oliv. & Hiern (Compositae) on Nauplii Brine Shrimps and Cancer Cell Lines. Asian J. Biol. Sci 2024, 17, 291-306. https://doi.org/10.3923/ajbs.2024.291.306
AMA Style
Akinola
FT, Oriyomi
VO, Ogundele
SB, Adekola
MB, Agbedahunsi
JM, Babalola
OO. Metabolite Profiling and Cytotoxic Investigation of Senecio biafrae Oliv. & Hiern (Compositae) on Nauplii Brine Shrimps and Cancer Cell Lines. Asian Journal of Biological Sciences. 2024; 17(3): 291-306. https://doi.org/10.3923/ajbs.2024.291.306
Chicago/Turabian Style
Akinola, Funke, Toyin, Vincent Olumayowa Oriyomi, Seun Bayonle Ogundele, Mukaila Babatunde Adekola, Joseph Morounfolu Agbedahunsi, and Olusegun Olubunmi Babalola.
2024. "Metabolite Profiling and Cytotoxic Investigation of Senecio biafrae Oliv. & Hiern (Compositae) on Nauplii Brine Shrimps and Cancer Cell Lines" Asian Journal of Biological Sciences 17, no. 3: 291-306. https://doi.org/10.3923/ajbs.2024.291.306

This work is licensed under a Creative Commons Attribution 4.0 International License.

