First Report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) Causing Basal Stem Rot Disease on Sunflower in Bangladesh
| Received 07 Mar, 2024 |
Accepted 29 Jun, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Sunflower is a prime source of vegetable oils worldwide. However, these plants are vulnerable to several abiotic and biotic factors, which limit the production of the crops. The current study aimed to detect the fungal pathogen linked with basal stem rot disease of sunflowers, the effect of physical factors on fungal growth and an efficient disease management scheme including an eco-friendly approach. Materials and Methods: Sunflower stems with explicit basal rot symptoms were collected from the commercially cultivated field in the Manikganj District of Bangladesh from July, 2021 to June, 2022. A further experiment was conducted to isolate the basal stem rot fungal pathogen of sunflowers using tissue planting methods. Morphological and molecular techniques were applied to identify the causal organism. Koch’s postulates were performed to confirm the pathogenic nature of the fungus. The effect of fungal culture media, temperature, pH, bio-control agents and fungicides were evaluated against the mycelial growth of the fungus. Results: The fungus was identified as Agroathelia rolfsii (Sacc) Redhead and S.T. Mullineux (Anomorph: Sclerotium rolfsii Sacc) based on morphological and molecular characterization. A BLAST search showed the pathogenic fungus (accession number: PP577975.1) had above 95% sequence similarity with previously deposited sequences Agroathelia rolfsii (JN241559.1; MH256035.1; JN241555.1). The fungus was to thrive in all the tested culture media. The fungus preferred a wider temperature range (25-35°C). The alkaline pH conditions (8 and 9) were the most suitable for the fungus. Bio-control agent Trichoderma asperellum inhibited the mycelial growth of the fungus moderately in dual culture. Importantly, commercial fungicide carbendazim inhibited the vegetative growth of A. rolfsii completely under in vitro conditions. Conclusion: Basal stem rot of sunflowers caused by Agroathelia rolfsii, is a new record in Bangladesh. The higher temperatures and basic pH stimulate the growth and development of the fungus. Bio-control agent Trichoderma asperellum and fungicide carbendazim could be used to manage sunflower stem rot diseases.
| Copyright © 2024 Sikder et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Sunflower (Helianthus annuus L.) is an annual or perennial flowering plant belonging to the family Asteraceae and locally known as Surjyamukhi in Bangladesh. It has round flower heads that in combination with the ligules, look like the sun. Sunflower has relatively been proven as a good oil seed crop among the growers in Bangladesh. It is the second-most important oil crop in the world after soybeans and has the property to produce high-quality edible oil1. Sunflower is native to the North American region2. The center of origin of the sunflower in North America and the plant was primarily domesticated and cultivated by Native Americans3. The government of Bangladesh has to spend a large amount of foreign exchange for importing oilseeds and edible oils to mitigate the increasing demand for its population which valued USD1574 million for edible oils and USD354 million for oilseeds4.
Nowadays, sunflower cultivation is a promising opportunity for the agricultural sector of Bangladesh as it is leading the oil production sector. In 2021, the total cultivated area of oil seeds was about 1234969.1 acre and the production rate was almost 995543.1 metric ton5. As it is one of the principal sources of edible oil with a low cholesterol content it has become popular among the growers. Besides, in situations where cultivating other crops is challenging owing to climate concerns, sunflower is an excellent alternative6. Sunflower oil has become the second most important vegetable oil worldwide and is usually used for cooking and making margarine and preparing salad. Sunflower oil is considered a premium because of its low level of saturated fats, light color, good taste, mild flavor and ability to cook at high temperatures7.
Various biotic and abiotic factors are directly and indirectly responsible for the yield reduction of sunflowers worldwide and cause significant economic losses. Several fungal pathogens cause major diseases of the crop including Phomopsis stem canker, Sclerotinia wilt, Verticillium wilt, Downy mildew, Charcoal rot, Rust, Sclerotinia root rot, Basal stalk rot, Sclerotinia stem rot, Sclerotinia head rot and mid-stalk rot, Alternaria leaf blight, Botrytis head rot, leaf and stem spot, Phoma black stem, Rhizopus head rot, etc.8,9. Among all the diseases, stem rot or basal stalk rot disease occurs in a more destructive form and causes much mortality of the crop. Sclerotinia root rot, basal stalk rot, stem rot, head rot and mid stalk rot of sunflowers have been reported in Pakistan8, USA9, South Africa10, Korea11. Agroathelia rolfsii (Anamorph: Sclerotium rolfsii) is a soil-inhibiting fungus, also known to cause collar rot in sunflowers in Turkey12, stem rot in Italy13 and China14. Gulya et al.15 reported that Alternaria sp. causes seedling blight, spots on the stem, head rots and other foliar diseases in sunflowers. In Bangladesh, to accurately identify and classify sunflower diseases, a comprehensive study has been conducted using digital image processing and computer vision approaches16. However, there is no comprehensive study on basal stem rot disease of sunflowers in Bangladesh.
Considering a foreside fact, the present research work was undertaken to isolate and identify basal stem rot-causing fungal pathogens of sunflowers using classical taxonomy and molecular techniques; to test the pathogenicity of identified pathogenic fungus; to investigate the fungal biology and cultural conditions for the mycelial growth of the isolated and identified fungus; to assess an efficient disease management protocol to manage basal stem rot disease of sunflowers.
MATERIALS AND METHODS
Study area: The fieldwork of the recent investigation was conducted in a commercially cultivated sunflower field located in the Manikgonj District, Bangladesh from July, 2021 to June, 2022. The laboratory part of the research was carried out in the Mycology and Plant Pathology Lab, Department of Botany, Jahangirnagar University, Dhaka, Bangladesh. Pathogenicity was conducted in the glasshouse of the Botanical Garden, Jahangirnagar University, Dhaka, Bangladesh.
Sample collection and isolation of the fungus: Infected stems with characteristic disease symptoms were collected in a zipper poly bag from the sunflower growing field, in the Manikgonj District,
Bangladesh. The sample was washed with running tap water and dried on a double-layered filter paper. Then it was cut into small pieces and surface sterilized with 5% sodium hypochlorite (NaOCl) for 3 min followed by 3-5 times washing with sterilized distilled water. The samples were air-dried to remove the surface water and placed on sterilized filter paper. Then the fragments were transferred to a Potato Dextrose Agar (PDA) medium and incubated at 25±2°C for 3-5 days.
Identification of the fungal pathogen: At first, the fungal isolates were identified based on morphological characterization. To do this, various cultural and morphological features viz., colony color, colony appearance, mycelial growth and branching pattern, mycelial pigmentation, the shape of hyphae, sclerotia formation pattern, etc. were studied17.
Fungal genomic DNA was extracted using Maxwell® 16 LEV Plant DNA Kit (Model: AS1420, Origin: Promega, United States of America). The ITS (Internal transcribed spacer) region of the rDNA in selected isolates of the fungal pathogen was amplified by PCR using universal primers ITS4 (5-TCCTCCGCTTATTGATATGC-3) and ITS5 (5-GGAAGTAAAAGTCGTAACAAGG-3) as described by White et al.18. To amplify target regions, PCR was performed in a 25 μL reaction mixture consisting of 12.5 μL of a Hot Start Green Master Mix (dNTPs, Buffer, MgCl2, Taq Pol., Origin: Promega, Madison, Wisconsin, United States of America), 1 μL of a DNA template (25 ng/uL), forward and reverse primers (20 pMol) and 9.5 μL of water. The PCR cycles were 95°C for 3 min (95°C for 30 sec, 48°C for 30 sec and 72°C for 50s) 35 cycles, 72°C for 5min and 4°C on hold overnight. The PCR product was purified using SV Gel and PCR Clean-Up System (Promega, United States of America) and sent to FIRST BASE Laboratories Sdn Bhd (Kuala Lumpur, Malaysia) for sequencing. The phylogenetic tree was constructed using the MEGA-11 software.
Koch’s postulates: The pathogenicity test was conducted in the experimental site of Botanical Garden, JU and the Laboratory of Mycology and Plant Pathology, Department of Botany, Jahangirnagar University, Savar, Dhaka, Bangladesh. Seedlings were grown for 30 days in pots containing sterile soil and then the basal region of the plants was artificially injured using a sterile scalpel, sclerotium (50% plants) and mycelial plug (50% plants) added at the base region of the plant. Plants treated with PDA free of mycelium or sclerotium served as controls. All plants were covered with polyethylene bags to retain moisture conditions and kept in the experimental sites. After two weeks, basal stem rot symptoms appeared at the base of the plant and pathogens were reisolated from symptomatic plants.
Evaluation of the effects of culture media, temperature and pH on the fungal growth: Cultural media have a significant attribute to the growth and development of fungi. To evaluate the effects of culture media on the proliferation of the tested fungus, different fungal culture media viz., PDA-Potato Dextrose Agar; PSA-Potato Sucrose Agar; CaA-Carrot Agar; YEA-Yeast Extract Agar; SGA-Saboured Dextrose Agar; HPA-Honey Peptone Agar; HaA-Hansen’s Agar was used19. The experiment was conducted in a controlled environment condition.
Temperature has a great regulatory effect on fungal growth. An optimum temperature regime always plays a significant role in fungal proliferation. In this study, the effects of different temperature conditions viz., 15, 20, 25, 30 and 35°C on the growth and development of isolated fungus was investigated20.
In most cases, the fungus is very sensitive to the pH level of the substrate where it grows. The effect of pH on the growth and development of the fungal isolate was observed using a suitable growth medium. Different pH levels viz., 5.0, 6.0, 7.0, 8.0 and 9.0 were evaluated on the mycelial growth of fungus21.
In vitro evaluation of the efficacy of biological control agents and fungicides against the tested fungal growth: The effective use of antagonistic microorganisms against pathogenic fungi has become a promising and eco-friendly approach to disease management in the agricultural sector. In this experiment, three Trichoderma spp. (Trichoderma harzianum, Trichoderma erinaceum and Trichoderma asperellum) were employed to evaluate the effectiveness against the disease-causing fungal pathogen. Following the dual culture technique, an agar disk of 2 mm in diameter of both antagonistic and tested organisms was excised from the edge of the actively growing 7 days culture and inoculated opposite to 1cm away from the edge of the culture Petri plates22. After 7 days of post-incubation (dpi), data was recorded and percent growth inhibition was measured. Besides, the food poison technique was used to evaluate the impact of three different concentrations of three selected fungicides, namely-Kazim (carbendazim), Copper blue (copper oxychloride) and Amistar top (azoxystrobin and difenoconazole) on the vegetative growth of the targeted fungus23.
Statistical analysis: Mycelial growth was recorded at 7 days post incubation (dpi). Data generated during the experiment was analyzed using standard statistical analyzing tools viz., MS Excel and SPSS-16. Sequence data of the tested fungal isolate was assessed by using the nucleotide BLAST tool and MEGA-11 software.
RESULTS
Symptoms of basal stem rot disease of sunflower: Basal stem rot disease of sunflowers starts at the collar region of the stem of the plant and progresses upwards as well as downwards. The initial symptoms of the disease appeared when the humidity of the environment was reported suddenly elevated and the temperature was approximately 28-33°C before or during the flowering period. The infected plants can be marked by their sickly appearance (Fig. 1a). Plants dried up due to the disease infestation. The lower portion of the stem was covered with white fungal mycelium (Fig. 1b). Dark brown lesions appeared on the base of the stem near ground level, leading to withering. After a few days of appearance, a sign of sclerotia is formed around the infected areas. With the progression of disease development, purple-brown colored, drenched watery lesions formed at the base of the infected plants, which wrapped the stem (Fig. 1c). Afterward, the pith of the stem started decaying and the plant became vulnerable to the wind (Fig. 1d). Eventually, the plants died within several days. Sclerotia formed both inside and outside of the stem.
Morphological characteristics of the isolated fungus: A fungus was isolated from the basal stem rot disease symptom of sunflower. It was characterized as a very fast-growing fungus. On the PDA medium, it produced abundant mycelium of white and woolly appearance with a smooth margin, creamy white color was formed on the reverse side of the Petri dishes (Fig. 2a).

|

|
The upper surface of the colony was slightly elevated center and woolly appearance. Under the microscope, the mycelia of the fungus were hyaline, septate and profusely branched. Conidia were absent throughout the mycelia. Initially, white-colored abundant sclerotia formed on the PDA medium. Gradually, brown-colored sclerotia formed after 3-4 days of vegetative growth, which turned into black color finally (Fig. 2b-c). Abundant clamp connections were present in the fungal colony (Fig. 2d).
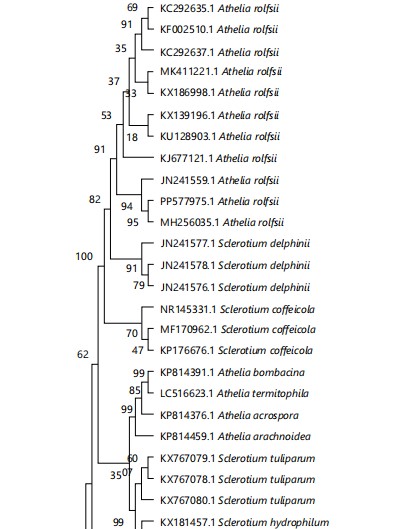
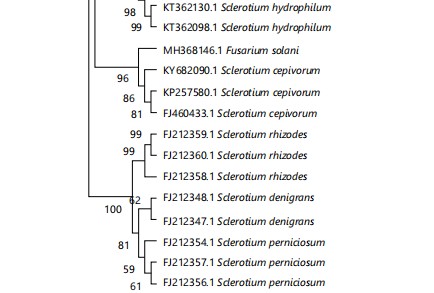
Molecular identification of the fungus: Based on the ITS-rDNA sequence data, the molecular analysis of the fungus was deduced. The length of the ITS sequence was approximately 679 bp. Once the generated sequence was suitably trimmed, it was uploaded to the NCBI database and received the accession number PP577975.1. The BLAST search was performed to retrieve the related sequences from the NCBI database and the sequences were aligned using MEGA-11 software, later phylogenetic tree was produced. In BLAST search, the studied fungus showed 94% to 99% similarity with ITS sequences of various strains of A. rolfsii (JN241559.1; MH256035.1 and JN241555.1) previously deposited in NCBI GenBank. In the phylogenetic analysis, the sequence alignment comprised 39 taxa of representative strains of Agroatheliaceae (Fig. 3), including our taxa-Agroathelia rolfsii and Fusarium solani as an outgroup. Maximum likelihood tree (ML), Neighbor-joining tree (NJ) and Maximum parsimony tree (MP) were generated and showed similar topology. The NJ tree has been presented here (Fig. 3).
The pathogenicity test following Koch’s postulates was successful. The fungal pathogen was reisolated and characterized properly to match the primary characterization of the fungus. This test confirmed the pathogenic nature of the fungus.
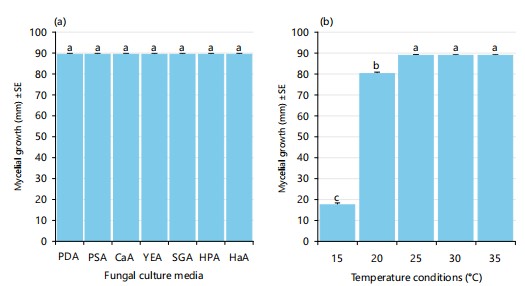
Effect of culture media, temperature and pH on mycelial growth of the Agroathelia rolfsii: Cultural media are the main factors to consider when studying the growth habits of fungi. In this research work, significant statistical differences among the culture media have not been found to support the growth of the tested fungus which indicates that the fungi prefer a wide range of hosts for their survival. On the other hand, being a soil-borne fungal pathogen it is very comfortable to grow on decayed organic matter and, thus grows equally on various fungal culture media as the media contain various types of organic substances. Maximum mycelial growth (90 mm) was obtained at all the tested culture media (Fig. 4). On PDA medium it produced a white, wooly and centrally emerged mycelia with a smooth margin which later formed a deep brown color sclerotium. In the case of PSA media, the fungus formed white, woolly mycelia with smooth margins and later formed light brown color sclerotium with a pearly appearance. On CaA and SGA media, the same mycelial appearance was observed but sclerotium formed scattered at the marginal region of Petri dishes. Besides, on YEA, HPA and HaA media, fungal growth was like the previously described media.
|
Fungal growth and development are significantly regulated by temperature, therefore there is always an optimum temperature at which fungi grow most effectively. Thus, the present study investigated how the temperature affects the vegetative growth of the fungus A. rolfsii on PDA media under in vitro conditions The experimental plates were incubated at five different temperatures viz., 15, 20, 25, 30 and 35°C, and optimistic results were found (Fig. 4-5).
|
The pathogenic fungus A. rolfsii exhibited the maximum (90 mm) vegetative growth at 25-35°C, besides, at 20 temperature the fungal growth was also moderately high (81.33 mm), however, the minimum mycelial growth of 17.88 mm was observed at the lowest temperature of 15°C (Fig. 4-5). Notably, there was no sclerotia formation at 15°C temperature whereas a very higher number of sclerotia was found at 35°C temperature, which indicates that temperature might play an important role in the formation, growth and development of the survival structure of the fungus (Fig. 4-5).
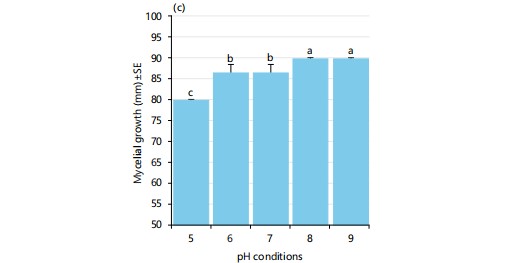
To investigate the effect of pH on the growth of A. rolfsii, five different pH conditions (pH 5, pH 6, pH 7, pH 8 and pH 9) have been experimented. It has been shown that pH is one of the important elements for exploring the ecology of spoilage fungi.

|
The variation in the pH level of PDA media correlates with the mycelial growth of almost all the fungi. The suitable pH of media for the best growth and development of test fungi was determined through this part of the experiment. The mycelial growth of Agroathelia rolfsii at various pH conditions was tested and promising results were found when the mycelial growth of the isolated pathogens was measured after 7 days of inoculation. There was a gradual increase of mycelial growth of Agroathelia rolfsii until pH 9, at pH 8 and 9 the mycelial growth was maximum (90 mm), followed by pH 6 and 7 (86.66 mm) and the lowest growth was observed at pH 5 (80 mm) (Fig. 4). It may be summarized that the fungus prefers a wide range of pH conditions. Neutral to slightly saline conditions were more suitable for the growth of Agroathelia rolfsii while acidic conditions may slow down the mycelial growth of the fungus (Fig. 5). We noticed that sclerotia formation was stimulated at pH 5 to 7 whereas there was no sclerotia formation at basic conditions (pH 8-9).
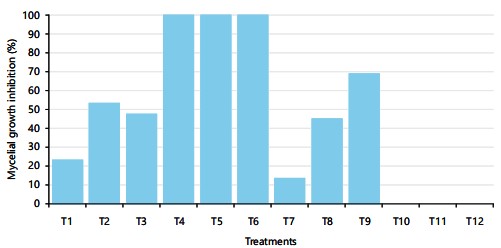
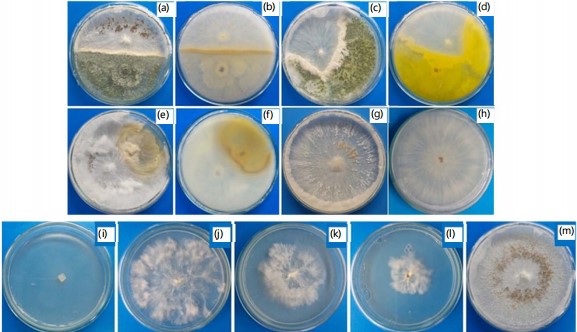
Efficacy of antagonistic fungi and fungicides against Agroathelia rolfsii: Trichoderma harzianum, Trichoderma erinaceum and Trichoderma asperellum were used as antagonistic organisms against the Agroathelia rolfsii at 25±2°C temperature. These three antagonistic fungi are used as biological control agents against the selected pathogenic fungus Agroathelia rolfsii of sunflower. Results indicate that green mold fungi were highly effective against the pathogenic fungus of sunflower. Trichoderma spp. showed promising results against the tested fungus (Fig. 6). During the dual culture of Agroathelia rolfsii and Trichoderma spp., Trichoderma asperellum. Trichoderma asperellum showed about 54% growth inhibition of pathogenic fungi followed by Trichoderma harzianum (47.5%) (Fig. 6). The lowest growth inhibition (23.5%) was found for Trichoderma erinaceum in the dual culture technique (Fig. 6).
Chemical fungicides are less preferable but effective means of plant disease control. In the present investigation, three commercially available chemical fungicides viz., Kazim (mancozeb), Amistar top (azoxystrobin and difenoconazole) and Copper blue (copper oxychloride) were used to evaluate the efficacy against the pathogenic fungus Agroathelia rolfsii. Kazim (mancozeb) at all the treated concentrations viz., 1000, 1500 and 2000 ppm exhibited very promising results i.e., 100% growth inhibition, whereas Amistar top at 2000 ppm concentration was found above 68% growth inhibition (Fig. 6). Importantly, the fungus could not form the sclerotia in the Amistar top-treated media. However, no growth inhibition was found for the application of copper oxychloride fungicide (Fig. 6).
|
DISCUSSIO N
The basal stem rot of sunflowers detected in a farmer’s field in Bangladesh. Using morphological and molecular techniques, a harmful fungal pathogen was identified as Agroaethelia rolfsii. Likewise, sunflower basal stem rot caused by A. rolfsii was reported in different countries worldwide such as Turkey12, Italy13, China14 and Egypt24. It is assumable that A. rolfsii causing sunflower basal stem rot is one of the economically significant fungal pathogens in Bangladesh. Importantly, this disease was most frequently observed in the farmer’s field during the rainy season in our country. In the phylogenetic tree, studied fungus A. rolfsii formed a cluster with numerous strains of the species A. rolfsii with 92% bootstrap value which confirmed the identity of the fungus at the species level. The ITS region of the nuclear rDNA can be used to study species-level relationships in fungi due to its higher degree of variation. Mahadevakumar et al.25 identified S. rolfsii associated with fruit rot disease of Cucurbita maxima using amplification and sequencing of the ITS region of the fungus. Similarly, Paul et al.26 studied the phylogenetic placement of Athelia rolfsii (Telomorph: Agroathelia rolfsii) causing blight disease of Ipomoea batatas in Korea, collar rot of sunflower in Turkey12 and stem rot of sunflower in China14.
All the tested fungal culture media were preferred by the fungus, which indicates they might use different sources of nutrients for their growth and development. Paul et al.27 studied the characterization of S. rolfsii causing root rot of sugar beet in Bangladesh. They observed that S. rolfsii grew well on PDA medium and OMA medium with an average radial growth rate of 2.3 and 2.1 mm/day, respectively. Sravani and Chandra28 found the PDA medium as a suitable culture medium for the best and optimum mycelial growth of S. rolfsii. Besides, Kushwaha et al.29 reported on the effect of different culture media on the vegetative growth and development of sclerotia formation of S. rolfsii Sacc. causing collar rot of lentils. Among the several culture media tested, PDA was the most suitable among the solid media for the proliferation and biomass production of this fungus. Present study suggests that A. rolfsii might have a wider host range because it grew in all studied fungal media.
Current study results agreed with the findings of Sravani and Chandra28 who studied the effect of temperature on the growth and development of S. rolfsii causing color rot of chickpea. A temperature of 30°C was proved to be the best and optimum for maximum mycelial growth of S. rolfsii. Paul et al.27 while studying the characterization of S. rolfsii causing root rot of sugar beet found 25°C as the optimum temperature for the fungal growth radial growth. Mukherjee and Raghu30 reported on how the antagonistic and biocontrol potentiality of Trichoderma sp. on S. rolfsii is manipulated by temperature. According to the experimental findings, S. rolfsii and Trichoderma sp. have distinct optimal growth temperatures: The pathogen prefers 30-35°C, whereas the antagonist prefers 25-30°C. Significantly, at 25°C and 30°C , Trichoderma overgrew S. rolfsii, but at 35°C and 37°C, S. rolfsii outgrew the Trichoderma colony. Kushwaha et al.29 reported on the effect of temperature on the growth and development of sclerotia formation of S. rolfsii causing collar rot in lentils and an excellent result was found. The results reveal that the growth of S. rolfsii was the maximum at 30°C, which was reduced significantly below 25°C. Current results suggest that A. rolfsii can grow in a wider range of temperatures and higher temperatures stimulate sclerotia formation.
Current results are supported by Sravani and Chandra28 who studied the pH factor affecting the growth and development of S. rolfsii causing color rot disease in chickpeas. The pH 6.0 was found to be most favorable for the fungal growth. Paul et al.27 while studying the characterization of S. rolfsii causing root rot of sugar beet observed the highest radial growth was favored pH 5.0-7.0 at a temperature of 25°C. Kushwaha et al.29 reported on the impact of pH on the mycelial growth and development of sclerotia formation of S. rolfsii causing collar rot in lentils. Among the several pH conditions, the highest mycelial formed at pH 6.5 while pH 6.0 was most suitable for sclerotia formation. Hence, current study results suggest that pH plays a vital role in the growth and development of sclerotia of A. rolfsii and, consequently, disease progression.
Recent results conformed with the finding of Nájera et al.31 who reported the maximum mycelial inhibition of S. rolfsii by Trichoderma virens strain G-41 (70.72%) and T. asperellum strain CSAEGro-1 (69%). Likewise, Jegathambigai et al.32 studied the effect of Trichoderma sp. on S. rolfsii, the causative agent of collar rot on Zamioculcas zamiifolia and an on-farm method to mass produce Trichoderma species. Significant growth inhibition of S. rolfsii was observed for all Trichoderma isolates tested in vitro. Out of all of these isolates, Trichoderma viride isolate TV1 was chosen for in vivo field trials as it exhibited the highest percentage of growth inhibition. Bosah et al.33 also reported in vitro microbial control of pathogenic S. rolfsii by Trichoderma sp. Hence, Trichoderma asperellum and T. harzianum may be an effective way of eco-friendly control measures for stem rot disease of sunflowers. However, further in vivo experiments needed to be conducted to confirm it.
Current study results conformed with the findings of Raghavendra and Srinivas34 who reported cent percent radial growth inhibition of S. rolfsii causing stem rot of groundnut by mancozeb@ 500, 1000, 1500, 2000 ppm concentration whereas copper oxychloride showed the least growth inhibition. Another lab study reported that mancozeb significantly inhibits the vegetative growth of S. rolfsii, responsible for causing collar rot disease in chickpeas under in vitro conditions, substantially controlling the growth of S. rolfsii under in vivo conditions35. Similarly, Shirsole et al.36 cited that the non-systemic fungicide mancozeb @100ppm concentration was found to be inhibitory against S. rolfsii causing collar rot of chickpea under in vitro conditions. Das et al.37 reported that mancozeb showed higher inhibitory effects against the fungal pathogen as compared to copper oxychloride. Similar results were reported by Prasad et al.38 who found 100% inhibition of S. rolfsii, the causal organism of tomato damping-off by mancozeb whereas 60% growth inhibition was recorded by copper oxychloride. Besides, Madhavi and Bhattiprolu39 cited that the combination of mancozeb and carbendazim was found to be very effective against S. rolfsii causing root-rot in chili. In another lab bioassay, while copper oxychloride was shown to be least effective against S. rolfsii even at higher doses, mancozeb was found to have a cent percent mycelial growth inhibition of S. rolfsii at all concentrations. Carbendazim 50 WP+mancozeb 75 WP (1:2 manual) exhibited cent percent growth inhibition of fungus at 500 ppm40. Moreover, mancozeb @ 0.25% was demonstrated to be effective in limiting 100% mycelial growth of S. rolfsii causing stem rot disease in groundnut41. Hence, carbendazim could be used to control the growth and development of A. rolfsii.
CONCLUSION
The basal stem rot has been considered as a major fungal disease of sunflower in Bangladesh. Results of the current research confirmed the identity of the disease-causing agent as Agroathelia rolfsii through morphological characterization and molecular study using ITS-rDNA sequence. Studies on the effect of physical parameters also revealed that temperature and pH have a great effect on fungal infestation. In the case of disease management strategy, the findings of the investigation revealed that fungal antagonist Trichoderma asperellum could be a promising source as a biological control agent and the chemical fungicide Kazim (carbendazim) could minimize the damage of disease at an economic level. Further work on the field trials of the biocontrol agents and synthetic fungicides against the pathogen will help to better understand the feasibility of the research.
SIGNIFICANCE STATEMENT
Sunflower is one of the principal sources of edible oil worldwide. However, the farmers often fail to obtain the expected yield due to heavy damage caused by fungal pathogens. Due to the harmful effects of chemical pesticides on the environment and human health, biological control is highly expected. The present study aimed to detect the causal organism of sunflower basal stem rot and to develop an eco-friendly disease management strategy. A causal fungal pathogen was identified manipulation of environmental factors on fungal growth was observed. Besides, bio-control agents were found to restrict the fungal growth under in vitro conditions. Moreover, carbendazim was a highly effective fungicide against the fungus. The findings of this research will contribute to managing the fungus for quality seed production of sunflowers.
REFERENCES
- Nasir Uddin, M., A. Sarker, I. Mahfuz and S.H.A. Mahdi, 2024. A study of abundance and diversity of flower-visiting insects on marigold (Tagetes erect L.) and sunflower (Helianthus annuus L.) at Rajshahi University Campus, Rajshahi, Bangladesh. J. Entomol. Zool. Stud., 12: 87-93.
- Yadava, D.K., S. Vasudev, N. Singh, T. Mohapatra and K.V. Prabhu, 2011. Breeding Major Oil Crops: Present Status and Future Research Needs. In: Technological Innovations in Major World Oil Crops, Volume 1: Breeding, Gupta, S.K. (Ed.), Springer, New York, ISBN 978-1-4614-0356-2, pp: 17-51.
- Gulya, T., R. Harveson, F. Mathew, C. Block and S. Thompson et al., 2018. Comprehensive disease survey of U.S. sunflower: Disease trends, research priorities and unanticipated impacts. Plant Dis., 103: 601-618.
- Miah, M.A.M. and M.R.I. Mondal, 2017. Oilseeds sector of Bangladesh: Challenges and opportunities. SAARC J. Agric., 15: 161-172.
- Sohel, M.H., A.A. Ratul, U.H. Hashi, M.F. Ali, T. Tasmim, M. Abdur Razzak, M. Mahabubur Rahman, 2023. Yield, profitability and prospects of sunflower cultivation in Chapainawabganj District Bangladesh. EBAUB J., 5: 37-45.
- Gupta, M.K., 2014. Sunflower oil and its applications. Lipid Technol., 26: 260-263.
- Martínez-Force, E., J.J. Salas and N.T. Dunford, 2015. Sunflower: Chemistry, Production, Processing, and Utilization. Elsevier Science, USA, ISBN: 9781630670627, Pages: 728.
- Qamar, M.I., M.U. Ghazanfar and M.I. Hamid, 2018. Identification of charcoal rot infecting pathogen of sunflower from Pakistan and detection of resistance source. Mycopathologia, 16: 15-22.
- Mukhtar, I., 2009. Sunflower disease and insect pests in Pakistan: A review. Afr. Crop Sci. J., 17: 109-118.
- Seiler, G.J., C.G. Misar, T.J. Gulya, W.R. Underwood, B.C. Flett, M.A. Gilley and S.G. Markell, 2017. Identification of novel sources of resistance to Sclerotinia basal stalk rot in South African sunflower germplasm. Plant Health Prog., 18: 87-90.
- Kwon, J.H., 2010. Occurrence of stem rot of sunflower (Helianthus annuus) caused by Sclerotium rolfsii. Res. Plant Dis., 16: 323-325.
- Cer, C. and A.U. Morca, 2020. First report of Athelia rolfsii (Sclerotium rolfsii Sacc.) causing collar rot disease on sunflower in Turkey. J. Plant Pathol., 102: 931-931.
- Infantino, A., G. di Giambattista and S. Socciarelli, 1997. First report of Sclerotium rolfsii on sunflower in Italy. Plant Dis., 81: 960-960.
- Zhang, T., Y. Li, G. Wen, J. Zou and J. Yang, 2023. First report of Athelia rolfsii causing stem rot on sunflower in Sichuan, China. Plant Dis., 107: 566-566.
- Gulya, T., K.Y. Rashid and S.M. Masirevic, 1997. Sunflower Diseases. In: Sunflower Technology and Production, Schneiter, A.A. (Ed.), Soil Science Society of America, Inc., Madison, Wisconsin, ISBN: 9780891182276, pp: 263-379.
- Sara, U., A. Rajbongshi, R. Shakil, B. Akter, S. Sazzad and M.S. Uddin, 2022. An extensive sunflower dataset representation for successful identification and classification of sunflower diseases. Data Brief, 42.
- Watanabe, T., 2002. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. 2nd Edn., CRC Press, Boca Raton, Florida, ISBN: 9780429075049, Pages: 504.
- White, T.J., T. Bruns, S. Lee and J. Taylor, 1990. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: PCR Protocols: A Guide to Methods and Applications, Innis, M.A., D.H. Gelfand, J.J. Sninsky and T.J. White (Eds.), Academic Press, Elsevier Inc., ISBN: 9780123721808, pp: 315-322.
- Sikder, M.M., M.S. Ahmmed, A. Sultana and N. Alam, 2021. First report on black spot disease of Phyllanthus emblica L. fruits caused by Thielaviopsis paradoxa in Bangladesh. Int. J. Agric. Res. Innovation Technol., 10: 38-46.
- Sultana, S., M.M. Sikder, F.A. Neela and N. Alam, 2023. First report of strawberry leaf blight caused by Fusarium fujikuroi species complex in Bangladesh. J. Bio-Sci., 30: 11-20.
- Sultana, A., M.M. Sikder and N. Alam, 2023. Identification and fungal biology of post-harvest fungi associated with amla fruits and it's in vitro control measures. Adv. Zool. Bot., 11: 1-11.
- Sultana, S., M.M. Sikder, M.S. Ahm and N. Alam, 2022. Neopestalotiopsis chrysea causing leaf spot disease of strawberry plants in Bangladesh. J. Plant Sci., 17: 66-74.
- Ahmmed, M.S., M.M. Sikder, S.R. Sarker, M.S. Hossain, R.D. Shubhra and N. Alam, 2022. First report of Pseudopestalotiopsis theae causing Aloe vera leaf spot disease in Bangladesh. Am. J. Plant Sci., 13: 193-204.
- EL-Ashmony, R., 2021. Biological and chemical control of sunflower basal stem rot caused by Sclerotium rolfsii. Sci. J. Agric. Sci., 3: 195-203.
- Mahadevakumar, S., V. Yadav, G.S. Tejaswini and G.R. Janardhana, 2016. Morphological and molecular characterization of Sclerotium rolfsii associated with fruit rot of Cucurbita maxima. Eur. J. Plant Pathol., 145: 215-219.
- Paul, N.C., E.J. Hwang, S.S. Nam, H.U. Lee and J.S. Lee et al., 2017. Phylogenetic placement and morphological characterization of Sclerotium rolfsii (Teleomorph: Athelia rolfsii) associated with blight disease of Ipomoea batatas in Korea. Mycobiology, 45: 129-138.
- Paul, S.K., N.U. Mahmud, D.R. Gupta, M.Z. Surovy, M. Rahman and M.T. Islam, 2021. Characterization of Sclerotium rolfsii causing root rot of sugar beet in Bangladesh. Sugar Tech, 23: 1199-1205.
- Sravani, B. and R. Chandra, 2020. Influence of media, pH and temperature on the growth of Sclerotium rolfsii (Sacc.) causing collar rot of chickpea. J. Pharmacogn. Phytochem., 9: 174-178.
- Kushwaha, S.K., S. Kumar, B. Chaudhary and R. Sahu, 2019. Effect of different media, pH and temperature on growth and sclerotia formation of Sclerotium rolfsii Sacc. causing collar rot of lentil. Chem. Sci. Rev. Lett., 8: 1-5.
- Mukherjee, P.K. and K. Raghu, 1997. Effect of temperature on antagonistic and biocontrol potential of shape Trichoderma sp. on Sclerotium rolfsii. Mycopathologia, 139: 151-155.
- Nájera, J.F.D., J.S. Castellanos, M.V. Hernández, S.A. Serna, O.G.A. Gómez, C.V. Verduzco and M.A. Ramos, 2018. Diagnosis and integrated management of fruit rot in Cucurbita argyrosperma, caused by Sclerotium rolfsii. Plant Pathol. J., 34: 171-181.
- Jegathambigai, V., R.S.W. Wijeratnam and R.L.C. Wijesundera, 2010. Effect of Trichoderma sp. on Sclerotium rolfsii, the causative agent of collar rot on Zamioculcas zamiifolia and an on farm method to mass produce Trichoderma species. Plant Pathol. J., 9: 47-55.
- Bosah, O., C.A. Igeleke and V.I. Omorusi, 2010. In vitro microbial control of pathogenic Sclerotium rolfsii. Int. J. Agric. Biol., 12: 474-476.
- Raghavendra, B. and T. Srinivas, 2020. In vitro studies on the effect of different fungicides against mycelial growth of Sclerotium rolfsii, the causal agent of stem rot in groundnut. Andhra Pradesh J. Agric. Sci., 6: 29-35.
- Khan, I.H. and A. Javaid, 2015. Chemical control of collar rot disease of chickpea. Pak. J. Phytopathol., 27: 61-68.
- Shirsole, S.S., N. Khare, N. Lakpale and A.S. Kotasthane, 2019. Evaluation of fungicides against Sclerotium rolfsii Sacc. incitant of collar rot of chickpea. Pharma Innovation J., 8: 310-316.
- Das, N.C., B.K. Dutta and D.C. Ray, 2014. Potential of some fungicides on the growth and development of Sclerotium rolfsii Sacc. in vitro. Int. J. Sci. Res. Publ., 4.
- Prasad, M.R., B.V. Sagar, G.U. Devi and S.R.K. Rao, 2017. In vitro evaluation of fungicides and biocontrol agents against damping off disease caused by Sclerotium rolfsii on tomato. Int. J. Pure Appl. Biosci., 5: 1247-1257.
- Madhavi, G.B. and S.L. Bhattiprolu, 2011. Integrated disease management of dry root rot of chilli incited by Sclerotium rolfsii (SACC). Intl. J. Plant Anim. Environ. Sci., 1: 31-37.
- Mehan, V.K., C.D. Mayee, D. McDonald, N. Ramakrishna and S. Jayanthi, 1995. Resistance in groundnut to Sclerotium rolfsii-caused stem and pod rot. Int. J. Pest Manage., 41: 79-83.
- Arunasri, P., B. Padmodaya, A. Prasanthi, M.V.S. Naidu and R.S. Tirumala et al., 2021. Impact of integrated disease management practices on soil health and disease incidence of stem rot of groundnut incited by Sclerotium rolfsii Sacc. Pharma Innovation, 10: 1349-1357.
How to Cite this paper?
APA-7 Style
Sikder,
M.M., Ahmmed,
M.S., Akter,
P., Shetu,
F.A., Akhter,
B., Alam,
N.B., Bhowmik,
D.D., Alam,
M.N. (2024). First Report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) Causing Basal Stem Rot Disease on Sunflower in Bangladesh. Asian Journal of Biological Sciences, 17(3), 448-461. https://doi.org/10.3923/ajbs.2024.448.461
ACS Style
Sikder,
M.M.; Ahmmed,
M.S.; Akter,
P.; Shetu,
F.A.; Akhter,
B.; Alam,
N.B.; Bhowmik,
D.D.; Alam,
M.N. First Report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) Causing Basal Stem Rot Disease on Sunflower in Bangladesh. Asian J. Biol. Sci 2024, 17, 448-461. https://doi.org/10.3923/ajbs.2024.448.461
AMA Style
Sikder
MM, Ahmmed
MS, Akter
P, Shetu
FA, Akhter
B, Alam
NB, Bhowmik
DD, Alam
MN. First Report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) Causing Basal Stem Rot Disease on Sunflower in Bangladesh. Asian Journal of Biological Sciences. 2024; 17(3): 448-461. https://doi.org/10.3923/ajbs.2024.448.461
Chicago/Turabian Style
Sikder, Md., Maniruzzaman, Md. Sabbir Ahmmed, Popy Akter, Farhana Akther Shetu, Beauty Akhter, Nusrat Binte Alam, Durga Das Bhowmik, and Md. Nuhu Alam.
2024. "First Report of Agroathelia rolfsii (Sclerotium rolfsii Sacc.) Causing Basal Stem Rot Disease on Sunflower in Bangladesh" Asian Journal of Biological Sciences 17, no. 3: 448-461. https://doi.org/10.3923/ajbs.2024.448.461

This work is licensed under a Creative Commons Attribution 4.0 International License.



